Inherited Proteoglycan Biosynthesis Defects-Current Laboratory Tools and Bikunin as a Promising Blood Biomarker
- PMID: 34828260
- PMCID: PMC8625474
- DOI: 10.3390/genes12111654
Inherited Proteoglycan Biosynthesis Defects-Current Laboratory Tools and Bikunin as a Promising Blood Biomarker
Abstract
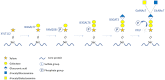
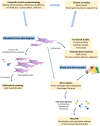
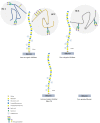
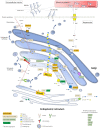
Proteoglycans consist of proteins linked to sulfated glycosaminoglycan chains. They constitute a family of macromolecules mainly involved in the architecture of organs and tissues as major components of extracellular matrices. Some proteoglycans also act as signaling molecules involved in inflammatory response as well as cell proliferation, adhesion, and differentiation. Inborn errors of proteoglycan metabolism are a group of orphan diseases with severe and irreversible skeletal abnormalities associated with multiorgan impairments. Identifying the gene variants that cause these pathologies proves to be difficult because of unspecific clinical symptoms, hardly accessible functional laboratory tests, and a lack of convenient blood biomarkers. In this review, we summarize the molecular pathways of proteoglycan biosynthesis, the associated inherited syndromes, and the related biochemical screening techniques, and we focus especially on a circulating proteoglycan called bikunin and on its potential as a new biomarker of these diseases.
Keywords: CDG; GAG; bikunin; linkeropathies; proteoglycans; skeletal dysplasia.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
Serum bikunin is a biomarker of linkeropathies.Clin Chim Acta. 2018 Oct;485:178-180. doi: 10.1016/j.cca.2018.06.044. Epub 2018 Jun 30. Clin Chim Acta. 2018. PMID: 29969625 No abstract available.
-
Serum bikunin isoforms in congenital disorders of glycosylation and linkeropathies.J Inherit Metab Dis. 2020 Nov;43(6):1349-1359. doi: 10.1002/jimd.12291. Epub 2020 Aug 7. J Inherit Metab Dis. 2020. PMID: 32700771
-
Structural analysis of bikunin glycosaminoglycan.J Am Chem Soc. 2008 Feb 27;130(8):2617-25. doi: 10.1021/ja0778500. Epub 2008 Feb 5. J Am Chem Soc. 2008. PMID: 18247611 Free PMC article.
-
Inter-alpha-trypsin inhibitor proteoglycan family--a group of proteins binding and stabilizing the extracellular matrix.Eur J Biochem. 1998 Mar 15;252(3):339-46. doi: 10.1046/j.1432-1327.1998.2520339.x. Eur J Biochem. 1998. PMID: 9546647 Review.
-
Mass spectrometry for congenital disorders of glycosylation, CDG.J Chromatogr B Analyt Technol Biomed Life Sci. 2006 Jun 21;838(1):3-8. doi: 10.1016/j.jchromb.2006.02.028. Epub 2006 Mar 6. J Chromatogr B Analyt Technol Biomed Life Sci. 2006. PMID: 16517226 Review.
Cited by
-
Glycosaminoglycan linkage region of urinary bikunin as a potentially useful biomarker for β3GalT6-deficient spondylodysplastic Ehlers-Danlos syndrome.JIMD Rep. 2022 Jun 28;63(5):462-467. doi: 10.1002/jmd2.12311. eCollection 2022 Sep. JIMD Rep. 2022. PMID: 36101818 Free PMC article.
-
Diagnostic and Therapeutic Approaches in Congenital Disorders of Glycosylation.Handb Exp Pharmacol. 2025;288:211-241. doi: 10.1007/164_2025_745. Handb Exp Pharmacol. 2025. PMID: 40119203 Review.
-
Mucopolysaccharidoses: Cellular Consequences of Glycosaminoglycans Accumulation and Potential Targets.Int J Mol Sci. 2022 Dec 28;24(1):477. doi: 10.3390/ijms24010477. Int J Mol Sci. 2022. PMID: 36613919 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

