Trisomy of Human Chromosome 21 Orthologs Mapping to Mouse Chromosome 10 Cause Age and Sex-Specific Learning Differences: Relevance to Down Syndrome
- PMID: 34828303
- PMCID: PMC8618694
- DOI: 10.3390/genes12111697
Trisomy of Human Chromosome 21 Orthologs Mapping to Mouse Chromosome 10 Cause Age and Sex-Specific Learning Differences: Relevance to Down Syndrome
Abstract
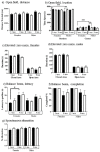
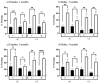
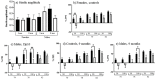
Down syndrome (DS), trisomy of human chromosome 21 (Hsa21), is the most common genetic cause of intellectual disability. The Dp10(1)Yey (Dp10) is a mouse model of DS that is trisomic for orthologs of 25% of the Hsa21 protein-coding genes, the entirety of the Hsa21 syntenic region on mouse chromosome 10. Trisomic genes include several involved in brain development and function, two that modify and regulate the activities of sex hormones, and two that produce sex-specific phenotypes as null mutants. These last four are the only Hsa21 genes with known sexually dimorphic properties. Relatively little is known about the potential contributions to the DS phenotype of segmental trisomy of Mmu10 orthologs. Here, we have tested separate cohorts of female and male Dp10 mice, at 3 and 9 months of age, in an open field elevated zero maze, rotarod, and balance beam, plus the learning and memory tasks, spontaneous alternation, puzzle box, double-H maze, context fear conditioning, and acoustic startle/prepulse inhibition, that depend upon the function of the prefrontal cortex, striatum, hippocampus, and cerebellum. We show that there are age and sex-specific differences in strengths and weaknesses, suggesting that genes within the telomere proximal region of Hsa21 influence the DS phenotype.
Keywords: Dp10(1)Yey; aging; double-H maze; female; hippocampus; intellectual disability; prefrontal cortex; prepulse inhibition; puzzle box; striatum.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
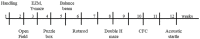



References
-
- Channell M.M., Mattie L.J., Hamilton D.R., Capone G.T., Mahone E.M., Sherman S.L., Rosser T.C., Reeves R.H., Kalb L.G. Down Syndrome Cognition Project. Capturing cognitive and behavioral variability among individuals with Down syndrome: A latent profile analysis. J. Neurodev. Disord. 2021;13:16. doi: 10.1186/s11689-021-09365-2. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

