DYRK1A Overexpression in Mice Downregulates the Gonadotropic Axis and Disturbs Early Stages of Spermatogenesis
- PMID: 34828406
- PMCID: PMC8621272
- DOI: 10.3390/genes12111800
DYRK1A Overexpression in Mice Downregulates the Gonadotropic Axis and Disturbs Early Stages of Spermatogenesis
Abstract
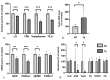
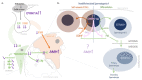
Down syndrome (DS) is the most common chromosomal disorder. It is responsible for intellectual disability (ID) and several medical conditions. Although men with DS are thought to be infertile, some spontaneous paternities have been reported. The few studies of the mechanism of infertility in men with DS are now dated. Recent research in zebrafish has indicated that overexpression of DYRK1A (the protein primarily responsible for ID in DS) impairs gonadogenesis at the embryonic stage. To better ascertain DYRK1A's role in infertility in DS, we investigated the effect of DYRK1A overexpression in a transgenic mouse model. We found that overexpression of DYRK1A impairs fertility in transgenic male mice. Interestingly, the mechanism in mice differs slightly from that observed in zebrafish but, with disruption of the early stages of spermatogenesis, is similar to that seen in humans. Unexpectedly, we observed hypogonadotropic hypogonadism in the transgenic mice.
Keywords: DYRK1A; Down syndrome; infertility.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

