Microbiological Quality and Resistance to an Artificial Gut Environment of Two Probiotic Formulations
- PMID: 34829062
- PMCID: PMC8617924
- DOI: 10.3390/foods10112781
Microbiological Quality and Resistance to an Artificial Gut Environment of Two Probiotic Formulations
Abstract
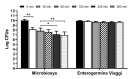
The quality control of probiotic products is the focus of numerous organizations worldwide. Several studies have highlighted the poor microbiological quality of many commercial probiotic formulations in terms of the identity of the contained microorganisms, viability, and purity, thus precluding the expected health benefits and representing a potential health risk for consumers. In this paper, we analyzed the contents of two probiotic formulations, one composed of an encapsulated mixture of lactobacilli and bifidobacteria, and one by a lyophilized yeast. The microorganisms contained in the products were quantified and identified using up-to-date methodologies, such as MALDI-TOF MS and metagenomic analysis. Moreover, as acid and bile tolerance is included among the criteria used to select probiotic microorganisms, in vitro tests were performed to evaluate the behavior of the formulations in conditions mimicking the harsh gastric environment and the intestinal fluids. Our results indicate the high quality of the formulations in terms of the enumeration and identification of the contained organisms, as well as the absence of contaminants. Moreover, both products tolerated the acidic conditions well, with encapsulation providing further protection for the microorganisms. A good tolerance to the simulated artificial intestinal conditions was also evidenced for both preparations.
Keywords: gastrointestinal behavior; identification; probiotics; quality; viable cells.
Conflict of interest statement
E.G. received research grants and/or travel grants and honoraria for speaking or participation at meetings from Novartis, OFF Italia, Polichem, and Sanofi S.r.l.; and R.L. is an employee of Sanofi Consumer Health Care, UK. This does not alter her adherence to food policies on sharing data and materials. D.M., F.C., M.C., and A.P. have no competing interests to declare.
Figures

References
-
- Hill C., Guarner F., Reid G., Gibson G.R., Merenstein D.J. Expert consensus document. The international scientific association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. - DOI - PubMed
-
- Fredua-Agyeman M., Parab S., Gaisford S. Evaluation of commercial probiotic products. Br. J. Pharm. 2016;1:84–89. doi: 10.5920/bjpharm.2016.11. - DOI
-
- Ansari J.M., Colasacco C., Emmanouil E., Kohlhepp S., Harriott O. Strain-level diversity of commercial probiotic isolates of Bacillus, Lactobacillus, and Saccharomyces species illustrated by molecular identification and phenotypic profiling. PLoS ONE. 2019;14:e0213841. doi: 10.1371/journal.pone.0213841. - DOI - PMC - PubMed
-
- Lugli G.A., Mangifesta M., Mancabelli L., Milani C., Turroni F., Viappiani A., van Sinderen D., Ventura M. Compositional assessment of bacterial communities in probiotic supplements by means of metagenomic techniques. Int. J. Food Microbiol. 2019;294:1–9. doi: 10.1016/j.ijfoodmicro.2019.01.011. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

