Aspergillus fumigatus Fumagillin Contributes to Host Cell Damage
- PMID: 34829223
- PMCID: PMC8619997
- DOI: 10.3390/jof7110936
Aspergillus fumigatus Fumagillin Contributes to Host Cell Damage
Abstract
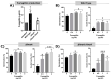
The activity of fumagillin, a mycotoxin produced by Aspergillus fumigatus, has not been studied in depth. In this study, we used a commercial fumagillin on cultures of two cell types (A549 pneumocytes and RAW 264.7 macrophages). This toxin joins its target, MetAP2 protein, inside cells and, as a result, significantly reduces the electron chain activity, the migration, and the proliferation ability on the A549 cells, or affects the viability and proliferation ability of the RAW 264.7 macrophages. However, the toxin stimulates the germination and double branch hypha production of fungal cultures, pointing out an intrinsic resistant mechanism to fumagillin of fungal strains. In this study, we also used a fumagillin non-producer A. fumigatus strain (∆fmaA) as well as its complemented strain (∆fmaA::fmaA) and we tested the fumagillin secretion of the fungal strains using an Ultra High-Performance Liquid Chromatography (UHPLC) method. Furthermore, fumagillin seems to protect the fungus against phagocytosis in vitro, and during in vivo studies using infection of immunosuppressed mice, a lower fungal burden in the lungs of mice infected with the ∆fmaA mutant was demonstrated.
Keywords: A549; Aspergillus fumigatus; RAW 264.7; UHPLC; fumagillin; mice infection; pathogenesis; virulence factor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
Grants and funding
LinkOut - more resources
Full Text Sources

