Novel Nile Blue Analogue Stains Yeast Vacuolar Membrane, Endoplasmic Reticulum, and Lipid Droplets, Inducing Cell Death through Vacuole Membrane Permeabilization
- PMID: 34829259
- PMCID: PMC8623074
- DOI: 10.3390/jof7110971
Novel Nile Blue Analogue Stains Yeast Vacuolar Membrane, Endoplasmic Reticulum, and Lipid Droplets, Inducing Cell Death through Vacuole Membrane Permeabilization
Abstract
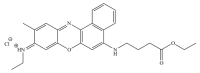
Phenoxazine derivatives such as Nile Blue analogues are assumed to be increasingly relevant in cell biology due to their fluorescence staining capabilities and antifungal and anticancer activities. However, the mechanisms underlying their effects remain poorly elucidated. Using S. cerevisiae as a eukaryotic model, we found that BaP1, a novel 5- and 9-N-substituted benzo[a]phenoxazine synthesized in our laboratory, when used in low concentrations, accumulates and stains the vacuolar membrane and the endoplasmic reticulum. In contrast, at higher concentrations, BaP1 stains lipid droplets and induces a regulated cell death process mediated by vacuolar membrane permeabilization. BaP1 also induced mitochondrial fragmentation and depolarization but did not lead to ROS accumulation, changes in intracellular Ca2+, or loss of plasma membrane integrity. Additionally, our results show that the cell death process is dependent on the vacuolar protease Pep4p and that the vacuole permeabilization results in its translocation from the vacuole to the cytosol. In addition, although nucleic acids are commonly described as targets of benzo[a]phenoxazines, we did not find any alterations at the DNA level. Our observations highlight BaP1 as a promising molecule for pharmacological application, using vacuole membrane permeabilization as a targeted approach.
Keywords: Nile Blue analogue; benzo[a]phenoxazine derivative; cell death mechanism; vacuole/lysosome membrane permeabilization; yeast as a eukaryotic cell model.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










References
-
- Frade V.H.J., Gonçalves M.S.T., Moura J.C.V.P. Synthesis and fluorescence properties of side-chain carboxylated 5,9-diaminobenzo[a]phenoxazinium salts. Tetrahedron Lett. 2005;46:4949–4952. doi: 10.1016/j.tetlet.2005.05.105. - DOI
-
- Frade V.H.J., Gonçalves M.S.T., Coutinho P.J.G., Moura J.C.V.P. Synthesis and spectral properties of long-wavelength fluorescent dyes. J. Photochem. Photobiol. A Chem. 2007;185:220–230. doi: 10.1016/j.jphotochem.2006.06.013. - DOI
-
- Raju B.R., Garcia A.M.F., Costa A.L.S., Coutinho P.J.G., Gonçalves M.S.T. Synthesis of new benzo[a]phenoxazinium probes possessing carboxylic ester, hydroxyl and amino functional groups: Photophysical studies in dry ethanol and conjugation with CdTe quantum dots. Dye Pigment. 2014;110:203–213. doi: 10.1016/j.dyepig.2014.04.006. - DOI
-
- Firmino A.D.G., Gonçalves M.S.T. Bifunctionalised long-wavelength fluorescent probes for biological applications. Tetrahedron Lett. 2012;53:4946–4950. doi: 10.1016/j.tetlet.2012.07.004. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

