Clinical Management and Outcome of Grade III Pneumonitis after Chemoradioimmunotherapy for Inoperable Stage III Non-Small Cell Lung Cancer-A Prospective Longitudinal Assessment
- PMID: 34829315
- PMCID: PMC8619082
- DOI: 10.3390/diagnostics11111968
Clinical Management and Outcome of Grade III Pneumonitis after Chemoradioimmunotherapy for Inoperable Stage III Non-Small Cell Lung Cancer-A Prospective Longitudinal Assessment
Abstract
Background: Maintenance treatment with immune-checkpoint inhibition (ICI) has been shown to significantly improve patient prognosis after chemoradiotherapy (CRT) for inoperable stage III NSCLC. This survival advantage may be achieved at the expense of an increased probability for symptomatic pneumonitis as CRT as well as ICI treatment is associated with the risk of treatment-related pulmonary toxicity.
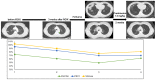
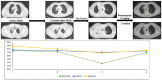
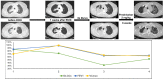
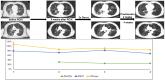
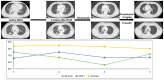
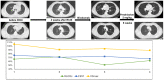
Methods: We screened a prospective chemoradioimmunotherapy (CRT-IO) cohort consisting of 38 patients and identified patients with therapy-related grade 3 pneumonitis. All patients were treated with intravenous high dose corticosteroids and closely monitored by CT-scans and extended longitudinal lung function tests. We analyzed lung function parameters and CT morphological features to characterize patients' outcome.
Results: Six (16%) patients treated with CRT-IO developed grade 3 pneumonitis one to six months after completion CRT. In the CT imaging, pneumonitis was characterized by diffuse ground glass capacities and in part pulmonary consolidations within and outside the planning target volume. Onset of pneumonitis was accompanied by a reduction in diffusion capacity in all cases. The mean decline of diffusion capacity was 25.8% [6-53%]. Under treatment with corticosteroids, all patients recovered regarding symptoms and changes in CT morphology. In five out of six patients, diffusion capacity improved to at least 80% of the baseline [80-96%]. One patient showed a significant increase of diffusion capacity after treatment (from 32% to 53%) but reached only 62% of the initial value.
Conclusions: Pneumonitis is a severe complication of CRT-IO. High-resolution CT imaging and extended lung function testing proved to be a suitable approach in detecting and monitoring of CRT-IO associated pneumonitis.
Keywords: NSCLC; chemoradioimmunotherapy; immunotherapy; lung function; pneumonitis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Radiation Versus Immune Checkpoint Inhibitor Associated Pneumonitis: Distinct Radiologic Morphologies.Oncologist. 2021 Oct;26(10):e1822-e1832. doi: 10.1002/onco.13900. Epub 2021 Aug 4. Oncologist. 2021. PMID: 34251728 Free PMC article.
-
PET-detected pneumonitis following curative-intent chemoradiation in non-small cell lung cancer (NSCLC): recognizing patterns and assessing the impact on the predictive ability of FDG-PET/CT response assessment.Eur J Nucl Med Mol Imaging. 2019 Aug;46(9):1869-1877. doi: 10.1007/s00259-019-04388-3. Epub 2019 Jun 12. Eur J Nucl Med Mol Imaging. 2019. PMID: 31190177
-
Safety evaluation of nivolumab added concurrently to radiotherapy in a standard first line chemo-radiotherapy regimen in stage III non-small cell lung cancer-The ETOP NICOLAS trial.Lung Cancer. 2019 Jul;133:83-87. doi: 10.1016/j.lungcan.2019.05.001. Epub 2019 May 3. Lung Cancer. 2019. PMID: 31200833 Clinical Trial.
-
Immune-Related Pneumonitis After Chemoradiotherapy and Subsequent Immune Checkpoint Blockade in Unresectable Stage III Non-Small-Cell Lung Cancer.Clin Lung Cancer. 2020 Sep;21(5):e435-e444. doi: 10.1016/j.cllc.2020.02.025. Epub 2020 Mar 9. Clin Lung Cancer. 2020. PMID: 32576443 Review.
-
Pulmonary toxicity of systemic lung cancer therapy.Respirology. 2020 Nov;25 Suppl 2:72-79. doi: 10.1111/resp.13915. Epub 2020 Jul 29. Respirology. 2020. PMID: 32729207 Review.
Cited by
-
Early Immune Checkpoint Inhibitor Administration Increases the Risk of Radiation-Induced Pneumonitis in Patients with Stage III Unresectable NSCLC Undergoing Chemoradiotherapy.Cancers (Basel). 2025 May 20;17(10):1711. doi: 10.3390/cancers17101711. Cancers (Basel). 2025. PMID: 40427209 Free PMC article.
-
Radiation-Induced Lung Injury: Prevention, Diagnostics and Therapy in the Era of the COVID-19 Pandemic.J Clin Med. 2022 Sep 27;11(19):5713. doi: 10.3390/jcm11195713. J Clin Med. 2022. PMID: 36233578 Free PMC article.
-
Comparison of post-chemoradiotherapy pneumonitis between Asian and non-Asian patients with locally advanced non-small cell lung cancer: a systematic review and meta-analysis.EClinicalMedicine. 2023 Sep 25;64:102246. doi: 10.1016/j.eclinm.2023.102246. eCollection 2023 Oct. EClinicalMedicine. 2023. PMID: 37781162 Free PMC article.
-
Dosimetric Predictors of Acute Radiation Pneumonitis and Esophagitis in Hypofractionated Thoracic Irradiation of Non-Small Cell Lung Cancer Patients With Poor Prognostic Factors.Adv Radiat Oncol. 2024 Nov 15;10(2):101682. doi: 10.1016/j.adro.2024.101682. eCollection 2025 Feb. Adv Radiat Oncol. 2024. PMID: 39896724 Free PMC article.
References
-
- Auperin A., Le Pechoux C., Rolland E., Curran W.J., Furuse K., Fournel P., Belderbos J., Clamon G., Ulutin H.C., Paulus R. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J. Clin. Oncol. 2010;28:2181–2190. doi: 10.1200/JCO.2009.26.2543. - DOI - PubMed
-
- Naidoo J., Nishino M., Patel S.P., Shankar B., Rekhtman N., Illei P., Camus P. Immune-Related Pneumonitis After Chemoradiotherapy and Subsequent Immune Checkpoint Blockade in Unresectable Stage III Non-Small-Cell Lung Cancer. Clin. Lung Cancer. 2020;21:e435–e444. doi: 10.1016/j.cllc.2020.02.025. - DOI - PubMed
-
- Paz-Ares L., Vicente D., Tafreshi A., Robinson A., Soto Parra H., Mazieres J., Hermes B., Cicin I., Medgyasszay B., Ro-driguez-Cid J., et al. A Randomized, Placebo-Controlled Trial of Pembrolizumab Plus Chemotherapy in Patients With Meta-static Squamous NSCLC: Protocol-Specified Final Analysis of KEYNOTE-407. J. Thorac. Oncol. 2020;15:1657–1669. doi: 10.1016/j.jtho.2020.06.015. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

