Comparative Analysis of Circulating Biomarkers for Patients Undergoing Resection of Colorectal Liver Metastases
- PMID: 34829346
- PMCID: PMC8622404
- DOI: 10.3390/diagnostics11111999
Comparative Analysis of Circulating Biomarkers for Patients Undergoing Resection of Colorectal Liver Metastases
Abstract
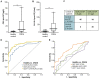
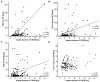
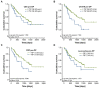
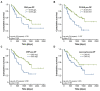
Surgical tumor resection has evolved as a potentially curative therapy for patients with resectable colorectal liver metastases (CRLM). However, disease recurrence is common and the available preoperative stratification strategies are often imprecise to identify the ideal candidates for surgical treatment, resulting in a postoperative 5-year survival rate below 50%. Data on the prognostic value of CEA, CA19-9 and other common laboratory parameters after CRLM resection are scarce and partly inconclusive. Here, we analyzed the prognostic potential of circulating CEA and CA19-9 in comparison to other standard laboratory markers in resectable CRLM patients. Serum levels of tumor markers and other laboratory parameters were analyzed in 125 patients with CRLM undergoing tumor resection at a tertiary referral center. Results were correlated with clinical data and outcome. Both tumor markers were significantly elevated in CRLM patients compared to healthy controls. Interestingly, elevated levels of CEA, CA19-9 and C-reactive protein (CRP) were associated with an unfavorable prognosis after CRLM resection in Kaplan-Meier curve analysis. However, only CEA and not CA19-9 or CRP serum levels were an independent prognostic marker in multivariate Cox regression analysis. Our data demonstrate that circulating levels of CEA rather than CA19-9 might be a valuable addition to the existing preoperative stratification algorithms to identify patients with a poor prognosis after CRLM resection.
Keywords: CA19-9; CEA; CRC; CRLM; CRP; cancer; liver resection; survival.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Van Cutsem E., Cervantes A., Adam R., Sobrero A., Van Krieken J.H., Aderka D., Aguilar E.A., Bardelli A., Benson A., Bodoky G., et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016;27:1386–1422. doi: 10.1093/annonc/mdw235. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

