A Strategy to Detect Emerging Non-Delta SARS-CoV-2 Variants with a Monoclonal Antibody Specific for the N501 Spike Residue
- PMID: 34829439
- PMCID: PMC8625484
- DOI: 10.3390/diagnostics11112092
A Strategy to Detect Emerging Non-Delta SARS-CoV-2 Variants with a Monoclonal Antibody Specific for the N501 Spike Residue
Abstract
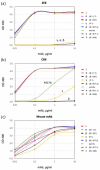
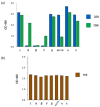
Efforts to control SARS-CoV-2 have been challenged by the emergence of variant strains that have important implications for clinical and epidemiological decision making. Four variants of concern (VOCs) have been designated by the Centers for Disease Control and Prevention (CDC), namely, B.1.617.2 (delta), B.1.1.7 (alpha), B.1.351 (beta), and P.1 (gamma), although the last three have been downgraded to variants being monitored (VBMs). VOCs and VBMs have shown increased transmissibility and/or disease severity, resistance to convalescent SARS-CoV-2 immunity and antibody therapeutics, and the potential to evade diagnostic detection. Methods are needed for point-of-care (POC) testing to rapidly identify these variants, protect vulnerable populations, and improve surveillance. Antigen-detection rapid diagnostic tests (Ag-RDTs) are ideal for POC use, but Ag-RDTs that recognize specific variants have not yet been implemented. Here, we describe a mAb (2E8) that is specific for the SARS-CoV-2 spike protein N501 residue. The 2E8 mAb can distinguish the delta VOC from variants with the N501Y meta-signature, which is characterized by convergent mutations that contribute to increased virulence and evasion of host immunity. Among the N501Y-containing mutants formerly designated as VOCs (alpha, beta, and gamma), a previously described mAb, CB6, can distinguish beta from alpha and gamma. When used in a sandwich ELISA, these mAbs sort these important SARS-CoV-2 variants into three diagnostic categories, namely, (1) delta, (2) alpha or gamma, and (3) beta. As delta is currently the predominant variant globally, they will be useful for POC testing to identify N501Y meta-signature variants, protect individuals in high-risk settings, and help detect epidemiological shifts among SARS-CoV-2 variants.
Keywords: COVID-19; OCMS™; SARS-CoV-2; clinical diagnostic test; delta variant; monoclonal antibody; variants of concern.
Conflict of interest statement
S.K.D., R.D.P., and F.H.A.-S. state a conflict of interest related to their role as inventors on a pending patent application filed by the Lankenau Institute for Medical Research. The other authors state no conflicts of interest for this work. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

Similar articles
-
Emergency SARS-CoV-2 Variants of Concern: Novel Multiplex Real-Time RT-PCR Assay for Rapid Detection and Surveillance.Microbiol Spectr. 2022 Feb 23;10(1):e0251321. doi: 10.1128/spectrum.02513-21. Epub 2022 Feb 23. Microbiol Spectr. 2022. PMID: 35196812 Free PMC article.
-
Real-Time RT-PCR Allelic Discrimination Assay for Detection of N501Y Mutation in the Spike Protein of SARS-CoV-2 Associated with B.1.1.7 Variant of Concern.Microbiol Spectr. 2022 Feb 23;10(1):e0068121. doi: 10.1128/spectrum.00681-21. Epub 2022 Feb 16. Microbiol Spectr. 2022. PMID: 35170989 Free PMC article.
-
CRISPR-Cas12a-Based Detection for the Major SARS-CoV-2 Variants of Concern.Microbiol Spectr. 2021 Dec 22;9(3):e0101721. doi: 10.1128/Spectrum.01017-21. Epub 2021 Nov 17. Microbiol Spectr. 2021. PMID: 34787487 Free PMC article.
-
SARS-CoV-2 Variants of Concern.Yonsei Med J. 2021 Nov;62(11):961-968. doi: 10.3349/ymj.2021.62.11.961. Yonsei Med J. 2021. PMID: 34672129 Free PMC article. Review.
-
SARS-CoV-2 Mutations and Their Impact on Diagnostics, Therapeutics and Vaccines.Front Med (Lausanne). 2022 Feb 22;9:815389. doi: 10.3389/fmed.2022.815389. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35273977 Free PMC article. Review.
Cited by
-
An Update on Detection Technologies for SARS-CoV-2 Variants of Concern.Viruses. 2022 Oct 22;14(11):2324. doi: 10.3390/v14112324. Viruses. 2022. PMID: 36366421 Free PMC article. Review.
-
Recombinant neutralizing secretory IgA antibodies for preventing mucosal acquisition and transmission of SARS-CoV-2.Mol Ther. 2024 Mar 6;32(3):689-703. doi: 10.1016/j.ymthe.2024.01.025. Epub 2024 Jan 24. Mol Ther. 2024. PMID: 38268188 Free PMC article.
-
COVI3D: Automatic COVID-19 CT Image-Based Classification and Visualization Platform Utilizing Virtual and Augmented Reality Technologies.Diagnostics (Basel). 2022 Mar 7;12(3):649. doi: 10.3390/diagnostics12030649. Diagnostics (Basel). 2022. PMID: 35328202 Free PMC article.
-
Application of the Nicoya OpenSPR to Studies of Biomolecular Binding: A Review of the Literature from 2016 to 2022.Sensors (Basel). 2023 May 17;23(10):4831. doi: 10.3390/s23104831. Sensors (Basel). 2023. PMID: 37430747 Free PMC article. Review.
-
QM/MM study of N501 involved intermolecular interaction between SARS-CoV-2 receptor binding domain and antibody of human origin.Comput Biol Chem. 2023 Feb;102:107810. doi: 10.1016/j.compbiolchem.2023.107810. Epub 2023 Jan 4. Comput Biol Chem. 2023. PMID: 36610304 Free PMC article.
References
-
- CDC SARS-CoV-2 Variant Classifications and Definitions. [(accessed on 22 October 2021)]; Available online: https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-info.html.
-
- Garcia-Beltran W.F., Lam E.C., Denis K.S., Nitido A.D., Garcia Z.H., Hauser B.M., Feldman J., Pavlovic M.N., Gregory D.J., Poznansky M.C., et al. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell. 2021;184:2372–2383.e9. doi: 10.1016/j.cell.2021.03.013. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

