Diagnostic Performance of Automated SARS-CoV-2 Antigen Assay in Nasal Swab during COVID-19 Vaccination Campaign
- PMID: 34829457
- PMCID: PMC8621910
- DOI: 10.3390/diagnostics11112110
Diagnostic Performance of Automated SARS-CoV-2 Antigen Assay in Nasal Swab during COVID-19 Vaccination Campaign
Abstract
Background: To control the spread of the pandemic brought about by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, it is necessary to have an automated reliable diagnostic assay. To date, the RT-PCR (RT-qPCR) has been the recommended laboratory method to diagnose SARS-CoV-2 infection, but there is a need for more automated and reliable tests. The aim of this real-life study was to assess the diagnostic performance of DiaSorin's LIAISON SARS-CoV-2 antigen (Ag) chemiluminescence immunoassay in detecting SARS-CoV-2 in vaccinated and unvaccinated individuals.
Methods: A prospective study was performed on 300 nasopharyngeal swabs randomly collected from 31 May to 6 July 2021. Nasopharyngeal samples were assayed with DiaSorin's LIAISON SARS-CoV-2 Ag and TaqPath™ COVID-19 multiplex RT-qPCR.
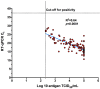
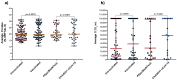
Results: Of 300 participants, 150 had a RT-qPCR confirmed SARS-CoV-2 infection of whom 113 (75.33%) were also detected by the DiaSorin LIAISON SARS-CoV-2 Ag. Taking RT-qPCR as a reference, the sensitivity and specificity of the DiaSorin LIAISON SARS-CoV-2 Ag assay were evaluated as 75.33% (95% CI = 67.64-82) and 100% (95% CI = 97.57-100), respectively. When a viral load cut-off was applied for high viral load (median cycle threshold (Ct) < 18.57), the overall sensitivity was increased to 96.55% (95% CI = 88.09-99.58). Interestingly, median RT-qPCR Ct and SARS-CoV-2 Ag values were similar between fully vaccinated and unvaccinated subjects.
Conclusions: Automated, quantitative LIAISON SARS-CoV-2 Ag assay shows good performance to identify SARS-CoV-2-infected individuals with moderate to high viral loads. LIAISON SARS-CoV-2 Ag testing could be used as frontline testing for COVID-19 diagnosis and be more suitable for large utilization.
Keywords: Ag-RDT; COVID-19; SARS-CoV2; diagnosis; rapid decisions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Systematic Review of Diagnostic Accuracy of DiaSorin Liaison SARS-CoV-2 Antigen Immunoassay.EJIFCC. 2022 Aug 8;33(2):94-104. eCollection 2022 Aug. EJIFCC. 2022. PMID: 36313904 Free PMC article. Review.
-
Comparison of performance of LIAISON SARS-CoV-2 antigen assay with RT-PCR during the Omicron wave.Acta Microbiol Immunol Hung. 2023 Jan 9;70(1):1-6. doi: 10.1556/030.2022.01863. Print 2023 Mar 2. Acta Microbiol Immunol Hung. 2023. PMID: 36622645
-
Clinical Assessment of the DiaSorin LIAISON SARS-CoV-2 Ag Chemiluminescence Immunoassay.EJIFCC. 2021 Jun 29;32(2):216-223. eCollection 2021 Jun. EJIFCC. 2021. PMID: 34421491 Free PMC article.
-
Evaluation of the Abbott Panbio™ COVID-19 antigen detection rapid diagnostic test among healthcare workers in elderly care.PLoS One. 2023 Feb 24;18(2):e0276244. doi: 10.1371/journal.pone.0276244. eCollection 2023. PLoS One. 2023. PMID: 36827362 Free PMC article.
-
Accuracy of rapid point-of-care antigen-based diagnostics for SARS-CoV-2: An updated systematic review and meta-analysis with meta-regression analyzing influencing factors.PLoS Med. 2022 May 26;19(5):e1004011. doi: 10.1371/journal.pmed.1004011. eCollection 2022 May. PLoS Med. 2022. PMID: 35617375 Free PMC article.
Cited by
-
Systematic Review of Diagnostic Accuracy of DiaSorin Liaison SARS-CoV-2 Antigen Immunoassay.EJIFCC. 2022 Aug 8;33(2):94-104. eCollection 2022 Aug. EJIFCC. 2022. PMID: 36313904 Free PMC article. Review.
-
Automated antigen assays display a high heterogeneity for the detection of SARS-CoV-2 variants of concern, including several Omicron sublineages.Med Microbiol Immunol. 2023 Oct;212(5):307-322. doi: 10.1007/s00430-023-00774-9. Epub 2023 Aug 10. Med Microbiol Immunol. 2023. PMID: 37561226 Free PMC article.
-
Analysis of diagnostic performance and factors causing nonspecific reactions in SARS-CoV-2 rapid antigen detection tests.J Infect Chemother. 2023 Feb;29(2):157-162. doi: 10.1016/j.jiac.2022.10.007. Epub 2022 Oct 23. J Infect Chemother. 2023. PMID: 36288777 Free PMC article.
-
High performance of the automated ADVIA Centaur Systems SARS-CoV-2 Antigen Assay in nasopharyngeal samples with high viral load.Int Microbiol. 2023 Aug;26(3):471-474. doi: 10.1007/s10123-022-00311-3. Epub 2022 Dec 17. Int Microbiol. 2023. PMID: 36527576 Free PMC article.
-
Correlation of COVID-19 vaccination and RT-PCR ct value among cases in Addis Ababa, Ethiopia: implication for future preparedness.BMC Infect Dis. 2024 Oct 9;24(1):1127. doi: 10.1186/s12879-024-10061-4. BMC Infect Dis. 2024. PMID: 39385106 Free PMC article.
References
-
- Kucharski A.J., Klepac P., Conlan A.J.K., Kissler S.M., Tang M.L., Fry H., Gog J.R., Edmunds W.J., Emery J.C., Medley G., et al. Effectiveness of isolation, testing, contact tracing, and physical distancing on reducing transmission of SARS-CoV-2 in different settings: A mathematical modelling study. Lancet Infect. Dis. 2020;20:1151–1160. doi: 10.1016/S1473-3099(20)30457-6. - DOI - PMC - PubMed
-
- Phua J., Weng L., Ling L., Egi M., Lim C.M., Divatia J.V., Shrestha B.R., Arabi Y.M., Ng J., Gomersall C.D., et al. Intensive care management of coronavirus disease 2019 (COVID-19): Challenges and recommendations. Lancet Respir. Med. 2020;8:506–517. doi: 10.1016/S2213-2600(20)30161-2. - DOI - PMC - PubMed
-
- Lefever S., Indevuyst C., Cuypers L., Dewaele K., Yin N., Cotton F., Padalko E., Oyaert M., Descy J., Cavalier E., et al. Comparison of the Quantitative DiaSorin Liaison Antigen Test to Reverse Transcription-PCR for the Diagnosis of COVID-19 in Symptomatic and Asymptomatic Outpatients. J. Clin. Microbiol. 2021;59:e0037421. doi: 10.1128/JCM.00374-21. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

