Diagnostic Performance of Automated SARS-CoV-2 Antigen Assay in Nasal Swab during COVID-19 Vaccination Campaign
- PMID: 34829457
- PMCID: PMC8621910
- DOI: 10.3390/diagnostics11112110
Diagnostic Performance of Automated SARS-CoV-2 Antigen Assay in Nasal Swab during COVID-19 Vaccination Campaign
Abstract
Background: To control the spread of the pandemic brought about by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, it is necessary to have an automated reliable diagnostic assay. To date, the RT-PCR (RT-qPCR) has been the recommended laboratory method to diagnose SARS-CoV-2 infection, but there is a need for more automated and reliable tests. The aim of this real-life study was to assess the diagnostic performance of DiaSorin's LIAISON SARS-CoV-2 antigen (Ag) chemiluminescence immunoassay in detecting SARS-CoV-2 in vaccinated and unvaccinated individuals.
Methods: A prospective study was performed on 300 nasopharyngeal swabs randomly collected from 31 May to 6 July 2021. Nasopharyngeal samples were assayed with DiaSorin's LIAISON SARS-CoV-2 Ag and TaqPath™ COVID-19 multiplex RT-qPCR.
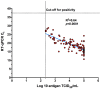
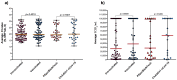
Results: Of 300 participants, 150 had a RT-qPCR confirmed SARS-CoV-2 infection of whom 113 (75.33%) were also detected by the DiaSorin LIAISON SARS-CoV-2 Ag. Taking RT-qPCR as a reference, the sensitivity and specificity of the DiaSorin LIAISON SARS-CoV-2 Ag assay were evaluated as 75.33% (95% CI = 67.64-82) and 100% (95% CI = 97.57-100), respectively. When a viral load cut-off was applied for high viral load (median cycle threshold (Ct) < 18.57), the overall sensitivity was increased to 96.55% (95% CI = 88.09-99.58). Interestingly, median RT-qPCR Ct and SARS-CoV-2 Ag values were similar between fully vaccinated and unvaccinated subjects.
Conclusions: Automated, quantitative LIAISON SARS-CoV-2 Ag assay shows good performance to identify SARS-CoV-2-infected individuals with moderate to high viral loads. LIAISON SARS-CoV-2 Ag testing could be used as frontline testing for COVID-19 diagnosis and be more suitable for large utilization.
Keywords: Ag-RDT; COVID-19; SARS-CoV2; diagnosis; rapid decisions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Kucharski A.J., Klepac P., Conlan A.J.K., Kissler S.M., Tang M.L., Fry H., Gog J.R., Edmunds W.J., Emery J.C., Medley G., et al. Effectiveness of isolation, testing, contact tracing, and physical distancing on reducing transmission of SARS-CoV-2 in different settings: A mathematical modelling study. Lancet Infect. Dis. 2020;20:1151–1160. doi: 10.1016/S1473-3099(20)30457-6. - DOI - PMC - PubMed
-
- Phua J., Weng L., Ling L., Egi M., Lim C.M., Divatia J.V., Shrestha B.R., Arabi Y.M., Ng J., Gomersall C.D., et al. Intensive care management of coronavirus disease 2019 (COVID-19): Challenges and recommendations. Lancet Respir. Med. 2020;8:506–517. doi: 10.1016/S2213-2600(20)30161-2. - DOI - PMC - PubMed
-
- Lefever S., Indevuyst C., Cuypers L., Dewaele K., Yin N., Cotton F., Padalko E., Oyaert M., Descy J., Cavalier E., et al. Comparison of the Quantitative DiaSorin Liaison Antigen Test to Reverse Transcription-PCR for the Diagnosis of COVID-19 in Symptomatic and Asymptomatic Outpatients. J. Clin. Microbiol. 2021;59:e0037421. doi: 10.1128/JCM.00374-21. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

