CRIF1 Deficiency Increased Homocysteine Production by Disrupting Dihydrofolate Reductase Expression in Vascular Endothelial Cells
- PMID: 34829516
- PMCID: PMC8614757
- DOI: 10.3390/antiox10111645
CRIF1 Deficiency Increased Homocysteine Production by Disrupting Dihydrofolate Reductase Expression in Vascular Endothelial Cells
Abstract
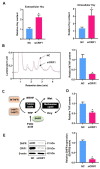
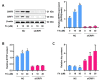
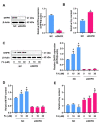
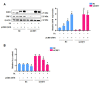
Elevated plasma homocysteine levels can induce vascular endothelial dysfunction; however, the mechanisms regulating homocysteine metabolism in impaired endothelial cells are currently unclear. In this study, we deleted the essential mitoribosomal gene CR6 interacting factor 1 (CRIF1) in human umbilical vein endothelial cells (HUVECs) and mice to induce endothelial cell dysfunction; then, we monitored homocysteine accumulation. We found that CRIF1 downregulation caused significant increases in intracellular and plasma concentrations of homocysteine, which were associated with decreased levels of folate cycle intermediates such as 5-methyltetrahydrofolate (MTHF) and tetrahydrofolate (THF). Moreover, dihydrofolate reductase (DHFR), a key enzyme in folate-mediated metabolism, exhibited impaired activity and decreased protein expression in CRIF1 knockdown endothelial cells. Supplementation with folic acid did not restore DHFR expression levels or MTHF and homocysteine concentrations in endothelial cells with a CRIF1 deletion or DHFR knockdown. However, the overexpression of DHFR in CRIF1 knockdown endothelial cells resulted in decreased accumulation of homocysteine. Taken together, our findings suggest that CRIF1-deleted endothelial cells accumulated more homocysteine, compared with control cells; this was primarily mediated by the disruption of DHFR expression.
Keywords: CR6 interacting factor 1; dihydrofolate reductase; folic acid; homocysteine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Graham I.M., Daly L.E., Refsum H.M., Robinson K., Brattstrom L.E., Ueland P.M., Palma-Reis R.J., Boers G.H., Sheahan R.G., Israelsson B., et al. Plasma homocysteine as a risk factor for vascular disease. The European Concerted Action Project. JAMA. 1997;277:1775–1781. doi: 10.1001/jama.1997.03540460039030. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

