Peucedanum japonicum Thunberg and Its Active Components Mitigate Oxidative Stress, Inflammation and Apoptosis after Urban Particulate Matter-Induced Ocular Surface Damage
- PMID: 34829588
- PMCID: PMC8614870
- DOI: 10.3390/antiox10111717
Peucedanum japonicum Thunberg and Its Active Components Mitigate Oxidative Stress, Inflammation and Apoptosis after Urban Particulate Matter-Induced Ocular Surface Damage
Abstract
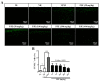
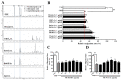
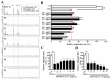
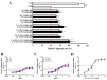
We previously demonstrated that urban particulate matter (UPM) exposure decreases the migration activity and survival of human corneal epithelial cells (HCECs). Herein, we investigated the potential to improve the corneal wound-healing ability of Peucedanum japonicum Thunb. leaf extract (PJE) and its active components on UPM-induced ocular surface damage in vitro and in vivo. PJE effectively assisted wound healing without altering HCEC survival and enhanced catalase (CAT), heme oxygenase 1 (HO1) and glutathione peroxidase 1 (GPX1) antioxidant gene expression. A corneal wound was uniformly induced on the right eye in all experimental animals and divided into eight groups such as two control groups (wounded right eye group-NR and non-wounded left eye group-NL), UPM treated group and PJEs (25, 50, 100, 200, 400 mg/kg) treated groups. Corneal abrasion model rats exposed to UPM showed delayed wound healing compared to unexposed rats, but wound healing was dose-dependently enhanced by PJE oral administration. Seventy-two hours after wound generation, inflammatory cells, apoptotic cells and interleukin-6 (IL-6) expression were increased substantially after UPM exposure, but PJE treatment significantly reduced the wound to an almost normal level while enhancing re-epithelialization without changing corneal thickness. Next, we tried to identify the key molecules for enhancing wound healing through fractionation. The major compounds in the fraction, confirmed by high-performance liquid chromatography (HPLC), were chlorogenic acid (CA), neochlorogenic acid (NCA) and cryptochlorogenic acid (CCA). Each type of CA isomers showed slightly different half maximal effective (EC50) and maximal effective (ECmax) concentrations, and their mixtures synergistically enhanced HCEC migration. Thus, corneal abrasion wound recovery after UPM exposure improved after PJE treatment, and the active PJE components were identified, providing an important basis to develop therapeutics for ocular surface damage using PJE.
Keywords: Peucedanum japonicum Thunberg; antioxidant; corneal epithelial cells; inflammation; urban particulate matter (UPM); wound healing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Tecer L.H., Alagha O., Karaca F., Tuncel G., Eldes N. Particulate matter (PM2.5, PM10-2.5, and PM10) and children’s hospital admissions for asthma and respiratory diseases: A bidirectional case-crossover study. J. Toxicol. Environ. Health Part A. 2008;71:512–520. doi: 10.1080/15287390801907459. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

