Calcium Dobesilate Modulates PKCδ-NADPH Oxidase- MAPK-NF-κB Signaling Pathway to Reduce CD14, TLR4, and MMP9 Expression during Monocyte-to-Macrophage Differentiation: Potential Therapeutic Implications for Atherosclerosis
- PMID: 34829669
- PMCID: PMC8615002
- DOI: 10.3390/antiox10111798
Calcium Dobesilate Modulates PKCδ-NADPH Oxidase- MAPK-NF-κB Signaling Pathway to Reduce CD14, TLR4, and MMP9 Expression during Monocyte-to-Macrophage Differentiation: Potential Therapeutic Implications for Atherosclerosis
Abstract
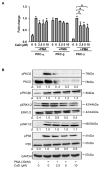
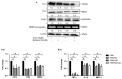
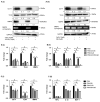
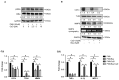
Monocyte-to-macrophage differentiation results in the secretion of various inflammatory mediators and oxidative stress molecules necessary for atherosclerosis pathogenesis. Consequently, this differentiation represents a potential clinical target in atherosclerosis. Calcium dobesilate (CaD), an established vasoactive and angioprotective drug in experimental models of diabetic microvascular complications reduces oxidative stress and inhibits inflammation via diverse molecular targets; however, its effect on monocytes/macrophages is poorly understood. In this study, we investigated the anti-inflammatory mechanism of CaD during phorbol 12-myristate 13-acetate (PMA)-induced monocyte-to-macrophage differentiation in in vitro models of sepsis (LPS) and hyperglycemia, using THP-1 monocytic cell line. CaD significantly suppressed CD14, TLR4, and MMP9 expression and activity, lowering pro-inflammatory mediators, such as IL1β, TNFα, and MCP-1. The effects of CaD translated through to studies on primary human macrophages. CaD inhibited reactive oxygen species (ROS) generation, PKCδ, MAPK (ERK1/2 and p38) phosphorylation, NOX2/p47phox expression, and membrane translocation. We used hydrogen peroxide (H2O2) to mimic oxidative stress, demonstrating that CaD suppressed PKCδ activation via its ROS-scavenging properties. Taken together, we demonstrate for the first time that CaD suppresses CD14, TLR4, MMP9, and signature pro-inflammatory cytokines, in human macrophages, via the downregulation of PKCδ/NADPH oxidase/ROS/MAPK/NF-κB-dependent signaling pathways. Our data present novel mechanisms of how CaD alleviates metabolic and infectious inflammation.
Keywords: PKCδ; atherosclerosis; calcium dobesilate; inflammation; monocyte-macrophage differentiation; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Curcuminoids Modulate the PKCδ/NADPH Oxidase/Reactive Oxygen Species Signaling Pathway and Suppress Matrix Invasion during Monocyte-Macrophage Differentiation.J Agric Food Chem. 2015 Oct 14;63(40):8838-48. doi: 10.1021/acs.jafc.5b04083. Epub 2015 Oct 5. J Agric Food Chem. 2015. PMID: 26414495
-
Thrombomodulin regulates monocye differentiation via PKCδ and ERK1/2 pathway in vitro and in atherosclerotic artery.Sci Rep. 2016 Dec 2;6:38421. doi: 10.1038/srep38421. Sci Rep. 2016. PMID: 27910925 Free PMC article.
-
Cinnamomum verum extract inhibits NOX2/ROS and PKCδ/JNK/AP-1/NF-κB pathway-mediated inflammatory response in PMA-stimulated THP-1 monocytes.Phytomedicine. 2023 Apr;112:154685. doi: 10.1016/j.phymed.2023.154685. Epub 2023 Jan 30. Phytomedicine. 2023. PMID: 36753827
-
SIRT1 inhibits differentiation of monocytes to macrophages: amelioration of synovial inflammation in rheumatoid arthritis.J Mol Med (Berl). 2016 Aug;94(8):921-31. doi: 10.1007/s00109-016-1402-7. Epub 2016 Mar 9. J Mol Med (Berl). 2016. PMID: 26956118
-
Role of TLR4/NADPH oxidase/ROS-activated p38 MAPK in VCAM-1 expression induced by lipopolysaccharide in human renal mesangial cells.Cell Commun Signal. 2012 Nov 15;10(1):33. doi: 10.1186/1478-811X-10-33. Cell Commun Signal. 2012. PMID: 23153039 Free PMC article.
Cited by
-
Macrophage-derived exosomes regulate gastric cancer cell oxaliplatin resistance by wrapping circ 0008253.Cell Cycle. 2023 Mar-Mar;22(6):705-717. doi: 10.1080/15384101.2022.2146839. Epub 2022 Nov 23. Cell Cycle. 2023. PMID: 36416404 Free PMC article.
-
Clinical application of calcium dobesilate in acute and sub-acute COVID-19: Two case reports.SAGE Open Med Case Rep. 2024 Mar 14;12:2050313X241236148. doi: 10.1177/2050313X241236148. eCollection 2024. SAGE Open Med Case Rep. 2024. PMID: 38495732 Free PMC article.
-
Endoplasmic reticulum stress contributes to cisplatin-induced chronic kidney disease via the PERK-PKCδ pathway.Cell Mol Life Sci. 2022 Jul 27;79(8):452. doi: 10.1007/s00018-022-04480-2. Cell Mol Life Sci. 2022. PMID: 35895146 Free PMC article.
-
Zinc-based Polyoxometalate Nanozyme Functionalized Hydrogels for optimizing the Hyperglycemic-Immune Microenvironment to Promote Diabetic Wound Regeneration.J Nanobiotechnology. 2024 Oct 8;22(1):611. doi: 10.1186/s12951-024-02840-7. J Nanobiotechnology. 2024. PMID: 39380018 Free PMC article.
-
Calcium dobesilate prevents PLD-induced hand-foot syndrome by alleviating capillary endothelial tight junction injury via the HA/CD44 pathway.Am J Cancer Res. 2023 Jul 15;13(7):3234-3245. eCollection 2023. Am J Cancer Res. 2023. PMID: 37559988 Free PMC article.
References
-
- Hermansson C., Lundqvist A., Magnusson L.U., Ullström C., Bergström G., Hultén L.M. Macrophage CD14 expression in human carotid plaques is associated with complicated lesions, correlates with thrombosis, and is reduced by angiotensin receptor blocker treatment. Int. Immunopharmacol. 2014;22:318–323. doi: 10.1016/j.intimp.2014.07.009. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

