Tumour Microenvironment Stress Promotes the Development of Drug Resistance
- PMID: 34829672
- PMCID: PMC8615091
- DOI: 10.3390/antiox10111801
Tumour Microenvironment Stress Promotes the Development of Drug Resistance
Abstract
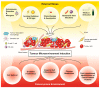
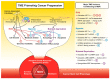
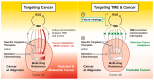
Multi-drug resistance (MDR) is a leading cause of cancer-related death, and it continues to be a major barrier to cancer treatment. The tumour microenvironment (TME) has proven to play an essential role in not only cancer progression and metastasis, but also the development of resistance to chemotherapy. Despite the significant advances in the efficacy of anti-cancer therapies, the development of drug resistance remains a major impediment to therapeutic success. This review highlights the interplay between various factors within the TME that collectively initiate or propagate MDR. The key TME-mediated mechanisms of MDR regulation that will be discussed herein include (1) altered metabolic processing and the reactive oxygen species (ROS)-hypoxia inducible factor (HIF) axis; (2) changes in stromal cells; (3) increased cancer cell survival via autophagy and failure of apoptosis; (4) altered drug delivery, uptake, or efflux and (5) the induction of a cancer stem cell (CSC) phenotype. The review also discusses thought-provoking ideas that may assist in overcoming the TME-induced MDR. We conclude that stressors from the TME and exposure to chemotherapeutic agents are strongly linked to the development of MDR in cancer cells. Therefore, there remains a vast area for potential research to further elicit the interplay between factors existing both within and outside the TME. Elucidating the mechanisms within this network is essential for developing new therapeutic strategies that are less prone to failure due to the development of resistance in cancer cells.
Keywords: cancer stem cells; drug resistance; reactive oxygen species; tumour microenvironmental stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
The biochemical and molecular mechanisms involved in the role of tumor micro-environment stress in development of drug resistance.Biochim Biophys Acta Gen Subj. 2019 Sep;1863(9):1390-1397. doi: 10.1016/j.bbagen.2019.06.007. Epub 2019 Jun 13. Biochim Biophys Acta Gen Subj. 2019. PMID: 31202693 Review.
-
Tumor microenvironment and epithelial mesenchymal transition as targets to overcome tumor multidrug resistance.Drug Resist Updat. 2020 Dec;53:100715. doi: 10.1016/j.drup.2020.100715. Epub 2020 Jun 20. Drug Resist Updat. 2020. PMID: 32679188 Review.
-
Induction of metastasis, cancer stem cell phenotype, and oncogenic metabolism in cancer cells by ionizing radiation.Mol Cancer. 2017 Jan 30;16(1):10. doi: 10.1186/s12943-016-0577-4. Mol Cancer. 2017. PMID: 28137309 Free PMC article. Review.
-
Modifying the tumour microenvironment and reverting tumour cells: New strategies for treating malignant tumours.Cell Prolif. 2020 Aug;53(8):e12865. doi: 10.1111/cpr.12865. Epub 2020 Jun 26. Cell Prolif. 2020. PMID: 32588948 Free PMC article. Review.
-
Tumor microenvironment diversity and plasticity in cancer multidrug resistance.Biochim Biophys Acta Rev Cancer. 2023 Nov;1878(6):188997. doi: 10.1016/j.bbcan.2023.188997. Epub 2023 Oct 12. Biochim Biophys Acta Rev Cancer. 2023. PMID: 37832894 Review.
Cited by
-
Targeting IL-8 and Its Receptors in Prostate Cancer: Inflammation, Stress Response, and Treatment Resistance.Cancers (Basel). 2024 Aug 8;16(16):2797. doi: 10.3390/cancers16162797. Cancers (Basel). 2024. PMID: 39199570 Free PMC article. Review.
-
In silico and in vitro analyses to investigate the effects of vitamin C on VEGF protein.J Taibah Univ Med Sci. 2024 Jul 10;19(4):775-789. doi: 10.1016/j.jtumed.2024.06.008. eCollection 2024 Aug. J Taibah Univ Med Sci. 2024. PMID: 39149519 Free PMC article.
-
The Effect of Radiotherapy on Cell Survival and Inflammatory Cytokine and Chemokine Secretion in a Co-Culture Model of Head and Neck Squamous Cell Carcinoma and Normal Cells.Biomedicines. 2023 Jun 20;11(6):1773. doi: 10.3390/biomedicines11061773. Biomedicines. 2023. PMID: 37371868 Free PMC article.
-
Targeting drug resistance in breast cancer: the potential of miRNA and nanotechnology-driven delivery systems.Nanoscale Adv. 2024 Nov 12;6(24):6079-6095. doi: 10.1039/d4na00660g. eCollection 2024 Dec 3. Nanoscale Adv. 2024. PMID: 39569336 Free PMC article. Review.
-
Constructing and identifying an eighteen-gene tumor microenvironment prognostic model for non-small cell lung cancer.World J Surg Oncol. 2024 Nov 28;22(1):319. doi: 10.1186/s12957-024-03588-y. World J Surg Oncol. 2024. PMID: 39609690 Free PMC article.
References
-
- Brown L.F., Guidi A.J., Schnitt S.J., van de Water L., Iruela-Arispe M.L., Yeo T.K., Tognazzi K., Dvorak H.F. Vascular stroma formation in carcinoma in situ, invasive carcinoma, and metastatic carcinoma of the breast. Clin. Cancer Res. 1999;5:1041–1056. - PubMed
-
- Aspord C., Pedroza-Gonzalez A., Gallegos M., Tindle S., Burton E.C., Su D., Marches F., Banchereau J., Palucka A.K. Breast cancer instructs dendritic cells to prime interleukin 13–secreting CD4+ T cells that facilitate tumor development. J. Exp. Med. 2007;204:1037–1047. doi: 10.1084/jem.20061120. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

