Antiangiogenic Properties of Axitinib versus Sorafenib Following Sunitinib Resistance in Human Endothelial Cells-A View towards Second Line Renal Cell Carcinoma Treatment
- PMID: 34829859
- PMCID: PMC8615644
- DOI: 10.3390/biomedicines9111630
Antiangiogenic Properties of Axitinib versus Sorafenib Following Sunitinib Resistance in Human Endothelial Cells-A View towards Second Line Renal Cell Carcinoma Treatment
Abstract
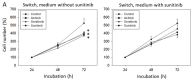
Tyrosine kinase inhibitors (TKIs) and immune checkpoint inhibitors predominate as first-line therapy options for renal cell carcinoma. When first-line TKI therapy fails due to resistance development, an optimal second-line therapy has not yet been established. The present investigation is directed towards comparing the anti-angiogenic properties of the TKIs, sorafenib and axitinib on human endothelial cells (HUVECs) with acquired resistance towards the TKI sunitinib. HUVECs were driven to resistance by continuously exposing them to sunitinib for six weeks. They were then switched to a 24 h or further six weeks treatment with sorafenib or axitinib. HUVEC growth, as well as angiogenesis (tube formation and scratch wound assay), were evaluated. Cell cycle proteins of the CDK-cyclin axis (CDK1 and 2, total and phosphorylated, cyclin A and B) and the mTOR pathway (AKT, total and phosphorylated) were also assessed. Axitinib (but not sorafenib) significantly suppressed growth of sunitinib-resistant HUVECs when they were exposed for six weeks. This axinitib-associated growth reduction was accompanied by a cell cycle block at the G0/G1-phase. Both axitinib and sorafenib reduced HUVEC tube length and prevented wound closure (sorafenib > axitinib) when applied to sunitinib-resistant HUVECs for six weeks. Protein analysis revealed diminished phosphorylation of CDK1, CDK2 and pAKT, accompanied by a suppression of cyclin A and B. Both drugs modulated CDK-cyclin and AKT-dependent signaling, associated either with both HUVEC growth and angiogenesis (axitinib) or angiogenesis alone (sorafenib). Axitinib and sorafenib may be equally applicable as second line treatment options, following sunitinib resistance.
Keywords: endothelial cells; renal cell carcinoma; resistance; second-line; tyrosine kinase inhibitors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Kroeger N., Choueiri T.K., Lee J.L., Bjarnason G.A., Knox J.J., MacKenzie M.J., Wood L., Srinivas S., Vaishamayan U.N., Rha S.Y., et al. Survival outcome and treatment response of patients with late relapse from renal cell carcinoma in the era of targeted therapy. Eur. Urol. 2014;65:1086–1092. doi: 10.1016/j.eururo.2013.07.031. - DOI - PubMed
-
- Aeppli S., Schmaus M., Eisen T., Escudier B., Grünwald V., Larkin J., McDermott D., Oldenburg J., Porta C., Rini B.I., et al. First-line treatment of metastatic clear cell renal cell carcinoma: A decision-making analysis among experts. ESMO Open. 2021;6:100030. doi: 10.1016/j.esmoop.2020.100030. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

