Role of Enteric Glia as Bridging Element between Gut Inflammation and Visceral Pain Consolidation during Acute Colitis in Rats
- PMID: 34829900
- PMCID: PMC8616000
- DOI: 10.3390/biomedicines9111671
Role of Enteric Glia as Bridging Element between Gut Inflammation and Visceral Pain Consolidation during Acute Colitis in Rats
Abstract
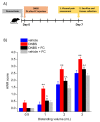
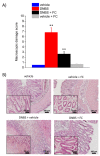
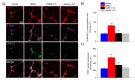
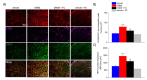
Acute inflammation is particularly relevant in the pathogenesis of visceral hypersensitivity associated with inflammatory bowel diseases. Glia within the enteric nervous system, as well as within the central nervous system, contributes to neuroplasticity during inflammation, but whether enteric glia has the potential to modify visceral sensitivity following colitis is still unknown. This work aimed to investigate the occurrence of changes in the neuron-glial networks controlling visceral perception along the gut-brain axis during colitis, and to assess the effects of peripheral glial manipulation. Enteric glia activity was altered by the poison fluorocitrate (FC; 10 µmol kg-1 i.p.) before inducing colitis in animals (2,4-dinitrobenzenesulfonic acid, DNBS; 30 mg in 0.25 mL EtOH 50%), and visceral sensitivity, colon damage, and glia activation along the pain pathway were studied. FC injection significantly reduced the visceral hyperalgesia, the histological damage, and the immune activation caused by DNBS. Intestinal inflammation is associated with a parallel overexpression of TRPV1 and S100β along the gut-brain axis (colonic myenteric plexuses, dorsal root ganglion, and periaqueductal grey area). This effect was prevented by FC. Peripheral glia activity modulation emerges as a promising strategy for counteracting visceral pain induced by colitis.
Keywords: S100β; TRPV1 receptors; astrocytes; dorsal root ganglion; enteric glia; inflammatory bowel disease; myenteric plexus; periaqueductal grey matter.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Endocannabinoids regulate enteric neuron-glia networks and visceral hypersensitivity following inflammation through a glial-dependent mechanism.Glia. 2024 Nov;72(11):2095-2114. doi: 10.1002/glia.24599. Epub 2024 Aug 12. Glia. 2024. PMID: 39132860
-
Anti-Hyperalgesic Efficacy of Acetyl L-Carnitine (ALCAR) Against Visceral Pain Induced by Colitis: Involvement of Glia in the Enteric and Central Nervous System.Int J Mol Sci. 2023 Oct 2;24(19):14841. doi: 10.3390/ijms241914841. Int J Mol Sci. 2023. PMID: 37834289 Free PMC article.
-
Enteric Glia: A New Player in Abdominal Pain.Cell Mol Gastroenterol Hepatol. 2019;7(2):433-445. doi: 10.1016/j.jcmgh.2018.11.005. Epub 2018 Nov 24. Cell Mol Gastroenterol Hepatol. 2019. PMID: 30739868 Free PMC article. Review.
-
High-fat diet impairs duodenal barrier function and elicits glia-dependent changes along the gut-brain axis that are required for anxiogenic and depressive-like behaviors.J Neuroinflammation. 2021 May 16;18(1):115. doi: 10.1186/s12974-021-02164-5. J Neuroinflammation. 2021. PMID: 33993886 Free PMC article.
-
Enteric glia as a player of gut-brain interactions during Parkinson's disease.Front Neurosci. 2023 Nov 1;17:1281710. doi: 10.3389/fnins.2023.1281710. eCollection 2023. Front Neurosci. 2023. PMID: 38027511 Free PMC article. Review.
Cited by
-
Metabotropic glutamate receptor-8 relieves neonatal maternal separation-induced visceral hypersensitivity in rats by regulating expression of TNF-α.Ann Transl Med. 2023 Jan 31;11(2):118. doi: 10.21037/atm-22-6452. Ann Transl Med. 2023. PMID: 36819583 Free PMC article.
-
Evaluation of the beneficial effects of a GABA-based product containing Melissa officinalis on post-inflammatory irritable bowel syndrome: a preclinical study.Front Pharmacol. 2024 Sep 20;15:1466824. doi: 10.3389/fphar.2024.1466824. eCollection 2024. Front Pharmacol. 2024. PMID: 39372212 Free PMC article.
-
Mini-review: Enteric glial cell reactions to inflammation and potential therapeutic implications for GI diseases, motility disorders, and abdominal pain.Neurosci Lett. 2023 Aug 24;812:137395. doi: 10.1016/j.neulet.2023.137395. Epub 2023 Jul 13. Neurosci Lett. 2023. PMID: 37451357 Free PMC article.
-
The Enteric Glia and Its Modulation by the Endocannabinoid System, a New Target for Cannabinoid-Based Nutraceuticals?Molecules. 2022 Oct 10;27(19):6773. doi: 10.3390/molecules27196773. Molecules. 2022. PMID: 36235308 Free PMC article. Review.
-
The enteric nervous system.Physiol Rev. 2023 Apr 1;103(2):1487-1564. doi: 10.1152/physrev.00018.2022. Epub 2022 Dec 15. Physiol Rev. 2023. PMID: 36521049 Free PMC article. Review.
References
-
- Miranda A., Nordstrom E., Mannem A., Smith C., Banerjee B., Sengupta J.N. The role of transient receptor potential vanilloid 1 in mechanical and chemical visceral hyperalgesia following experimental colitis. Neuroscience. 2007;148:1021–1032. doi: 10.1016/j.neuroscience.2007.05.034. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

