Oxidized LDL Increase the Proinflammatory Profile of Human Visceral Adipocytes Produced by Hypoxia
- PMID: 34829944
- PMCID: PMC8615639
- DOI: 10.3390/biomedicines9111715
Oxidized LDL Increase the Proinflammatory Profile of Human Visceral Adipocytes Produced by Hypoxia
Abstract
Background: Little is known about the effects of hypoxia on scavenger receptors (SRs) levels in adipocytes. We analyzed the effect of morbid obesity and hypoxia on SRs and inflammation markers in human visceral adipocytes and whether ox-LDL modify the inflammatory profile produced by hypoxia.
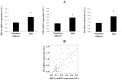
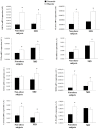
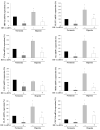
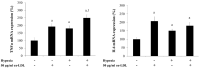
Methods: We studied in 17 non-obese and 20 subjects with morbid obesity (MO) the mRNA expression of HIF-1α, SRs (LOX-1, MSR1, CL-P1 and CXCL16), IL6 and TNFα in visceral adipocytes and the effect of hypoxia with or without ox-LDL on visceral in vitro-differentiated adipocytes (VDA).
Results: HIF-1α, TNFα, IL6, LOX-1, MSR1 and CXCL16 expression in adipocytes was increased in MO when compared with those in non-obese subjects (p < 0.05). The expression of most of the inflammatory markers and SRs gene correlated with HIF-1α. In VDA, hypoxia increased TNFα, IL6, MSR1, CXCL16 and CL-P1 (p < 0.05) in non-obese subjects, and TNFα, IL6, MSR1 and CXCL16 (p < 0.05) in MO. Silencing HIF-1α prevented the increase of TNFα, IL6, LOX-1, MSR1, CL-P1 and CXCL16 expression (p < 0.05). The combination of hypoxia and ox-LDL produced higher TNFα expression (p = 0.041).
Conclusions: Morbid obesity and hypoxia increased SRs and inflammatory markers in visceral adipocytes. In a hypoxic state, ox-LDL increased the proinflammatory response of visceral adipocytes to hypoxia.
Keywords: adipocytes; hypoxia; morbid obesity; oxidized LDL; scavenger receptors.
Conflict of interest statement
The authors declare that they have no conflicts of interests.
Figures



References
-
- García-Fuentes E., Santiago-Fernández C., Gutiérrez-Repiso C., Mayas M.D., Oliva-Olivera W., Coín-Aragüez L., Alcaide J., Ocaña-Wilhelmi L., Vendrell J., Tinahones F.J., et al. Hypoxia is associated with a lower expression of genes involved in lipogenesis in visceral adipose tissue. J. Transl. Med. 2015;13:373. doi: 10.1186/s12967-015-0732-5. - DOI - PMC - PubMed
-
- Fernández-Sánchez A., Madrigal-Santillán E., Bautista M., Esquivel-Soto J., Morales-González A., Esquivel-Chirino C., Durante-Montiel I., Sánchez-Rivera G., Valadez-Vega C., Morales-González J.A. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2011;12:3117–3132. doi: 10.3390/ijms12053117. - DOI - PMC - PubMed

