Study of the Role of the Tyrosine Kinase Receptor MerTK in the Development of Kidney Ischemia-Reperfusion Injury in RCS Rats
- PMID: 34829984
- PMCID: PMC8618874
- DOI: 10.3390/ijms222212103
Study of the Role of the Tyrosine Kinase Receptor MerTK in the Development of Kidney Ischemia-Reperfusion Injury in RCS Rats
Abstract
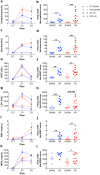
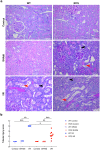
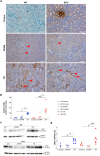
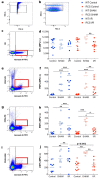
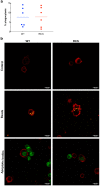
Renal ischaemia reperfusion (I/R) triggers a cascade of events including oxidative stress, apoptotic body and microparticle (MP) formation as well as an acute inflammatory process that may contribute to organ failure. Macrophages are recruited to phagocytose cell debris and MPs. The tyrosine kinase receptor MerTK is a major player in the phagocytosis process. Experimental models of renal I/R events are of major importance for identifying I/R key players and for elaborating novel therapeutical approaches. A major aim of our study was to investigate possible involvement of MerTK in renal I/R. We performed our study on both natural mutant rats for MerTK (referred to as RCS) and on wild type rats referred to as WT. I/R was established by of bilateral clamping of the renal pedicles for 30' followed by three days of reperfusion. Plasma samples were analysed for creatinine, aspartate aminotransferase (ASAT), lactate dehydrogenase (LDH), kidney injury molecule -1 (KIM-1), and neutrophil gelatinase-associated lipocalin (NGAL) levels and for MPs. Kidney tissue damage and CD68-positive cell requirement were analysed by histochemistry. monocyte chemoattractant protein-1 (MCP-1), myeloperoxidase (MPO), inducible nitric oxide synthase (iNOS), and histone 3A (H3A) levels in kidney tissue lysates were analysed by western blotting. The phagocytic activity of blood-isolated monocytes collected from RCS or WT towards annexin-V positive bodies derived from cultured renal cell was assessed by fluorescence-activated single cell sorting (FACS) and confocal microscopy analyses. The renal I/R model for RCS rat described for the first time here paves the way for further investigations of MerTK-dependent events in renal tissue injury and repair mechanisms.
Keywords: MerTK; RCS rats; inflammation; ischemia-reperfusion; microparticles; phagocytosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

