The Different Molecular Code in Generation of Dopaminergic Neurons from Astrocytes and Mesenchymal Stem Cells
- PMID: 34830023
- PMCID: PMC8622032
- DOI: 10.3390/ijms222212141
The Different Molecular Code in Generation of Dopaminergic Neurons from Astrocytes and Mesenchymal Stem Cells
Abstract
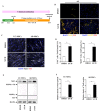
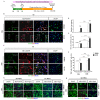
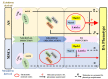
Transplantation of exogenous dopaminergic (DA) neurons is an alternative strategy to replenish DA neurons that have lost along the course of Parkinson's disease (PD). From the perspective of ethical acceptation, the source limitations, and the intrinsic features of PD pathology, astrocytes (AS) and mesenchymal stem cells (MSCs) are the two promising candidates of DA induction. In the present study, we induced AS or MSCs primary culture by the combination of the classical transcription-factor cocktails Mash1, Lmx1a, and Nurr1 (MLN), the chemical cocktails (S/C/D), and the morphogens SHH, FGF8, and FGF2 (S/F8/F2); the efficiency of induction into DA neurons was further analyzed by using immunostaining against the DA neuronal markers. AS could be efficiently converted into the DA neurons in vitro by the transcriptional regulation of MLN, and the combination with S/C/D or S/F8/F2 further increased the conversion efficiency. In contrast, MSCs from umbilical cord (UC-MSCs) or adipose tissue (AD-MSCs) showed moderate TH immunoreactivity after the induction with S/F8/F2 instead of with MLN or S/C/D. Our data demonstrated that AS and MSCs held lineage-specific molecular codes on the induction into DA neurons and highlighted the unique superiority of AS in the potential of cell replacement therapy for PD.
Keywords: AS; DA candidate; MSCs; PD; morphogens; small molecules; transcription factors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Conversion of human umbilical cord mesenchymal stem cells in Wharton's jelly to dopamine neurons mediated by the Lmx1a and neurturin in vitro: potential therapeutic application for Parkinson's disease in a rhesus monkey model.PLoS One. 2013 May 28;8(5):e64000. doi: 10.1371/journal.pone.0064000. Print 2013. PLoS One. 2013. Retraction in: PLoS One. 2020 Nov 2;15(11):e0242032. doi: 10.1371/journal.pone.0242032. PMID: 23724014 Free PMC article. Retracted.
-
Generation of functional dopaminergic neurons from human spermatogonial stem cells to rescue parkinsonian phenotypes.Stem Cell Res Ther. 2019 Jun 27;10(1):195. doi: 10.1186/s13287-019-1294-x. Stem Cell Res Ther. 2019. PMID: 31248447 Free PMC article.
-
The Effect of Human Umbilical Cord Mesenchymal Stromal Cells in Protection of Dopaminergic Neurons from Apoptosis by Reducing Oxidative Stress in the Early Stage of a 6-OHDA-Induced Parkinson's Disease Model.Cell Transplant. 2019 Dec;28(1_suppl):87S-99S. doi: 10.1177/0963689719891134. Epub 2019 Nov 28. Cell Transplant. 2019. PMID: 31775521 Free PMC article.
-
Manipulated mesenchymal stem cell therapy in the treatment of Parkinson's disease.Stem Cell Res Ther. 2024 Dec 18;15(1):476. doi: 10.1186/s13287-024-04073-9. Stem Cell Res Ther. 2024. PMID: 39696636 Free PMC article. Review.
-
Efficient induction of dopaminergic neurons from embryonic stem cells for application to Parkinson's disease.Yonsei Med J. 2004 Jun 30;45 Suppl:23-7. doi: 10.3349/ymj.2004.45.Suppl.23. Yonsei Med J. 2004. Retraction in: Yonsei Med J. 2004 Dec 31;45(6):1203. doi: 10.3349/ymj.2004.45.6.1203. PMID: 15250046 Retracted. Review.
Cited by
-
Mesenchymal Stem Cells Restore Endothelial Integrity and Alleviate Emotional Impairments in a Diabetic Mouse Model via Inhibition of MMP-9 Activity.Int J Mol Sci. 2025 Apr 3;26(7):3355. doi: 10.3390/ijms26073355. Int J Mol Sci. 2025. PMID: 40244194 Free PMC article.
-
Activation of angiopoietin-1 signaling with engineering mesenchymal stem cells promoted efficient angiogenesis in diabetic wound healing.Stem Cell Res Ther. 2025 Feb 21;16(1):75. doi: 10.1186/s13287-025-04207-7. Stem Cell Res Ther. 2025. PMID: 39985096 Free PMC article.
-
Cerebral-Organoid-Derived Exosomes Alleviate Oxidative Stress and Promote LMX1A-Dependent Dopaminergic Differentiation.Int J Mol Sci. 2023 Jul 4;24(13):11048. doi: 10.3390/ijms241311048. Int J Mol Sci. 2023. PMID: 37446226 Free PMC article.
References
-
- Freed W.J., Perlow M.J., Karoum F., Seiger A., Olson L., Hoffer B.J., Wyatt R.J. Restoration of Dopaminergic Function by Grafbng of Fetal Rat Substantia Nigra to the Caudate Nucleus: Long-Term Behavioral, Biochemical, and Histochemical Studies. Ann. Neurol. 1980;8:510–519. doi: 10.1002/ana.410080508. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- XDA16020807/Strategic Priority Research Program of the Chinese Academy of Sciences
- BE2018668/Key Research and Development Program of Jiangsu Province
- BE2017669/Key Research and Development Program of Jiangsu Province
- 81701332/National Natural Science Foundation of China
- 2019B020235001/Key Areas Research and Development Program of Guangdong
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

