Tau Cleavage Contributes to Cognitive Dysfunction in Strepto-Zotocin-Induced Sporadic Alzheimer's Disease (sAD) Mouse Model
- PMID: 34830036
- PMCID: PMC8618605
- DOI: 10.3390/ijms222212158
Tau Cleavage Contributes to Cognitive Dysfunction in Strepto-Zotocin-Induced Sporadic Alzheimer's Disease (sAD) Mouse Model
Abstract
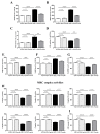
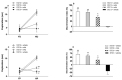
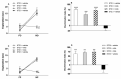
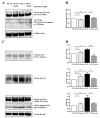
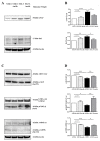
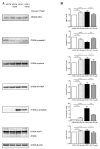
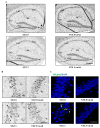
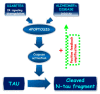
Tau cleavage plays a crucial role in the onset and progression of Alzheimer's Disease (AD), a widespread neurodegenerative disease whose incidence is expected to increase in the next years. While genetic and familial forms of AD (fAD) occurring early in life represent less than 1%, the sporadic and late-onset ones (sAD) are the most common, with ageing being an important risk factor. Intracerebroventricular (ICV) infusion of streptozotocin (STZ)-a compound used in the systemic induction of diabetes due to its ability to damage the pancreatic β cells and to induce insulin resistance-mimics in rodents several behavioral, molecular and histopathological hallmarks of sAD, including memory/learning disturbance, amyloid-β (Aβ) accumulation, tau hyperphosphorylation, oxidative stress and brain glucose hypometabolism. We have demonstrated that pathological truncation of tau at its N-terminal domain occurs into hippocampi from two well-established transgenic lines of fAD animal models, such as Tg2576 and 3xTg mice, and that it's in vivo neutralization via intravenous (i.v.) administration of the cleavage-specific anti-tau 12A12 monoclonal antibody (mAb) is strongly neuroprotective. Here, we report the therapeutic efficacy of 12A12mAb in STZ-infused mice after 14 days (short-term immunization, STIR) and 21 days (long-term immunization regimen, LTIR) of i.v. delivery. A virtually complete recovery was detected after three weeks of 12A12mAb immunization in both novel object recognition test (NORT) and object place recognition task (OPRT). Consistently, three weeks of this immunization regimen relieved in hippocampi from ICV-STZ mice the AD-like up-regulation of amyloid precursor protein (APP), the tau hyperphosphorylation and neuroinflammation, likely due to modulation of the PI3K/AKT/GSK3-β axis and the AMP-activated protein kinase (AMPK) activities. Cerebral oxidative stress, mitochondrial impairment, synaptic and histological alterations occurring in STZ-infused mice were also strongly attenuated by 12A12mAb delivery. These results further strengthen the causal role of N-terminal tau cleavage in AD pathogenesis and indicate that its specific neutralization by non-invasive administration of 12A12mAb can be a therapeutic option for both fAD and sAD patients, as well as for those showing type 2 diabetes as a comorbidity.
Keywords: cognitive/memory impairment; immunotherapy; neuroinflammation; non-transgenic Alzheimer’s Disease mouse model; oxidative stress; streptozotocin (STZ); tau cleavage.
Conflict of interest statement
Authors have no conflict of interest to be disclosed. All authors have seen and approved the manuscript.
Figures


References
-
- Corsetti V., Borreca A., Latina V., Giacovazzo G., Pignataro A., Krashia P., Natale F., Cocco S., Rinaudo M., Malerba F., et al. Passive immunotherapy for N-truncated tau ameliorates the cognitive deficits in two mouse Alzheimer’s disease models. Brain Commun. 2020;2:fcaa039. doi: 10.1093/braincomms/fcaa039. - DOI - PMC - PubMed
-
- Steen E., Terry B.M., Rivera E.J., Cannon J.L., Neely T.R., Tavares R., Xu X.J., Wands J.R., de la Monte S.M. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease—Is this type 3 diabetes? J. Alzheimer’s Dis. 2005;7:63–80. doi: 10.3233/JAD-2005-7107. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- (FOE D.M 865/2019)/Fondo Ordinario Enti (FOE D.M 865/2019) funds in the framework of a collaboration agreement between the Italian National Research Council and EBRI (2019-2021)
- Progetto T0002E0001 G04014_13_04_2021/Regione Lazio, POR FESR Lazio 2014-2020. "Progetti di Gruppi di Ricerca 2020" (Determinazione dirigenziale n.G08487 del 19 luglio 2020)
LinkOut - more resources
Full Text Sources
Medical

