The Effect of Tuberculosis Antimicrobials on the Immunometabolic Profiles of Primary Human Macrophages Stimulated with Mycobacterium tuberculosis
- PMID: 34830070
- PMCID: PMC8624646
- DOI: 10.3390/ijms222212189
The Effect of Tuberculosis Antimicrobials on the Immunometabolic Profiles of Primary Human Macrophages Stimulated with Mycobacterium tuberculosis
Abstract
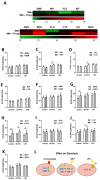
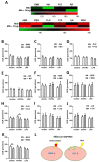
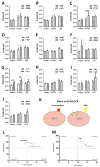
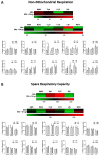
Tuberculosis (TB) remains a global health challenge. Patients with drug-sensitive and drug-resistant TB undergo long, arduous, and complex treatment regimens, often involving multiple antimicrobials. While these drugs were initially implemented based on their bactericidal effects, some studies show that TB antimicrobials can also directly affect cells of the immune system, altering their immune function. As use of these antimicrobials has been the mainstay of TB therapy for over fifty years now, it is more important than ever to understand how these antimicrobials affect key pathways of the immune system. One such central pathway, which underpins the immune response to a variety of infections, is immunometabolism, namely glycolysis and oxidative phosphorylation (OXPHOS). We hypothesise that in addition to their direct bactericidal effect on Mycobacterium tuberculosis (Mtb), current TB antimicrobials can modulate immunometabolic profiles and alter mitochondrial function in primary human macrophages. Human monocyte-derived macrophages (hMDMs) were differentiated from PBMCs isolated from healthy blood donors, and treated with four first-line and six second-line TB antimicrobials three hours post stimulation with either iH37Rv-Mtb or lipopolysaccharide (LPS). 24 h post stimulation, baseline metabolism and mitochondrial function were determined using the Seahorse Extracellular Flux Analyser. The effect of these antimicrobials on cytokine and chemokine production was also assayed using Meso Scale Discovery Multi-Array technology. We show that some of the TB antimicrobials tested can significantly alter OXPHOS and glycolysis in uninfected, iH37Rv-Mtb, and LPS-stimulated hMDMs. We also demonstrate how these antimicrobial-induced immunometabolic effects are linked with alterations in mitochondrial function. Our results show that TB antimicrobials, specifically clofazimine, can modify host immunometabolism and mitochondrial function. Moreover, clofazimine significantly increased the production of IL-6 in human macrophages that were stimulated with iH37Rv-Mtb. This provides further insight into the use of some of these TB antimicrobials as potential host-directed therapies in patients with early and active disease, which could help to inform TB treatment strategies in the future.
Keywords: antimicrobials; bioenergetics; drug-resistant tuberculosis; glycolysis; host-directed therapy; lipopolysaccharide; mitochondrial function; oxidative phosphorylation; tuberculosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Mitini-Nkhoma S.C., Chimbayo E.T., Mzinza D.T., Mhango D.V., Chirambo A.P., Mandalasi C., Lakudzala A.E., Tembo D.L., Jambo K.C., Mwandumba H.C. Something Old, Something New: Ion Channel Blockers as Potential Anti-Tuberculosis Agents. Front. Immunol. 2021;12:2203. doi: 10.3389/fimmu.2021.665785. - DOI - PMC - PubMed
-
- Hayford F.E.A., Dolman R.C., Blaauw R., Nienaber A., Smuts C.M., Malan L., Ricci C. The effects of anti-inflammatory agents as host-directed adjunct treatment of tuberculosis in humans: A systematic review and meta-analysis. Respir. Res. 2020;21:223. doi: 10.1186/s12931-020-01488-9. - DOI - PMC - PubMed
-
- Cox D.J., Coleman A.M., Gogan K.M., Phelan J.J., Maoldomhnaigh C.Ó., Dunne P.J., Basdeo S.A., Keane J. Inhibiting Histone Deacetylases in Human Macrophages Promotes Glycolysis, IL-1β, and T Helper Cell Responses to Mycobacterium tuberculosis. Front. Immunol. 2020;11:1609. doi: 10.3389/fimmu.2020.01609. - DOI - PMC - PubMed
-
- Cahill C., O’Connell F., Gogan K.M., Cox D.J., Basdeo S.A., O’Sullivan J., Gordon S.V., Keane J., Phelan J.J. The Iron Chelator Desferrioxamine Increases the Efficacy of Bedaquiline in Primary Human Macrophages Infected with BCG. Int. J. Mol. Sci. 2021;22:2938. doi: 10.3390/ijms22062938. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

