Dissection of the Regulatory Elements of the Complex Expression Pattern of Puckered, a Dual-Specificity JNK Phosphatase
- PMID: 34830088
- PMCID: PMC8623796
- DOI: 10.3390/ijms222212205
Dissection of the Regulatory Elements of the Complex Expression Pattern of Puckered, a Dual-Specificity JNK Phosphatase
Abstract
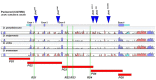
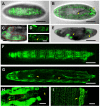
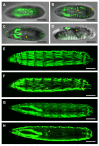
For developmental processes, we know most of the gene networks controlling specific cell responses. We still have to determine how these networks cooperate and how signals become integrated. The JNK pathway is one of the key elements modulating cellular responses during development. Yet, we still know little about how the core components of the pathway interact with additional regulators or how this network modulates cellular responses in the whole organism in homeostasis or during tissue morphogenesis. We have performed a promoter analysis, searching for potential regulatory sequences of puckered (puc) and identified different specific enhancers directing gene expression in different tissues and at different developmental times. Remarkably, some of these domains respond to the JNK activity, but not all. Altogether, these analyses show that puc expression regulation is very complex and that JNK activities participate in non-previously known processes during the development of Drosophila.
Keywords: Drosophila; JNK; dual specificity phosphatase; gene expression.
Conflict of interest statement
The authors declare no competing financial interests.
Figures






Similar articles
-
puckered encodes a phosphatase that mediates a feedback loop regulating JNK activity during dorsal closure in Drosophila.Genes Dev. 1998 Feb 15;12(4):557-70. doi: 10.1101/gad.12.4.557. Genes Dev. 1998. PMID: 9472024 Free PMC article.
-
The Drosophila DUSP puckered is phosphorylated by JNK and p38 in response to arsenite-induced oxidative stress.Biochem Biophys Res Commun. 2012 Feb 10;418(2):301-6. doi: 10.1016/j.bbrc.2012.01.015. Epub 2012 Jan 12. Biochem Biophys Res Commun. 2012. PMID: 22266315
-
Drosophila puckered regulates Fos/Jun levels during follicle cell morphogenesis.Development. 2001 May;128(10):1845-56. doi: 10.1242/dev.128.10.1845. Development. 2001. PMID: 11311164
-
Combinatorial transcriptional regulation: the interaction of transcription factors and cell signaling molecules with homeodomain proteins in Drosophila development.Crit Rev Eukaryot Gene Expr. 2001;11(1-3):145-71. Crit Rev Eukaryot Gene Expr. 2001. PMID: 11693959 Review.
-
Regulating cell morphogenesis: the Drosophila Jun N-terminal kinase pathway.Genesis. 2013 Mar;51(3):147-62. doi: 10.1002/dvg.22354. Epub 2012 Nov 20. Genesis. 2013. PMID: 23109363 Review.
Cited by
-
JNK signaling and integrins cooperate to maintain cell adhesion during epithelial fusion in Drosophila.Front Cell Dev Biol. 2024 Jan 9;11:1034484. doi: 10.3389/fcell.2023.1034484. eCollection 2023. Front Cell Dev Biol. 2024. PMID: 38264353 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

