Acetylenic Synthetic Betulin Derivatives Inhibit Akt and Erk Kinases Activity, Trigger Apoptosis and Suppress Proliferation of Neuroblastoma and Rhabdomyosarcoma Cell Lines
- PMID: 34830180
- PMCID: PMC8624615
- DOI: 10.3390/ijms222212299
Acetylenic Synthetic Betulin Derivatives Inhibit Akt and Erk Kinases Activity, Trigger Apoptosis and Suppress Proliferation of Neuroblastoma and Rhabdomyosarcoma Cell Lines
Abstract
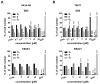
Neuroblastoma (NB) and rhabdomyosarcoma (RMS), the most common pediatric extracranial solid tumors, still represent an important clinical challenge since no effective treatment is available for metastatic and recurrent disease. Hence, there is an urgent need for the development of new chemotherapeutics to improve the outcome of patients. Betulin (Bet), a triterpenoid from the bark of birches, demonstrated interesting anti-cancer potential. The modification of natural phytochemicals with evidenced anti-tumor activity, including Bet, is one of the methods of receiving new compounds for potential implementation in oncological treatment. Here, we showed that two acetylenic synthetic Bet derivatives (ASBDs), EB5 and EB25/1, reduced the viability and proliferation of SK-N-AS and TE671 cells, as measured by MTT and BrdU tests, respectively. Moreover, ASBDs were also more cytotoxic than temozolomide (TMZ) and cisplatin (cis-diaminedichloroplatinum [II], CDDP) in vitro, and the combination of EB5 with CDDP enhanced anti-cancer effects. We also showed the slowdown of cell cycle progression at S/G2 phases mediated by EB5 using FACS flow cytometry. The decreased viability and proliferation of pediatric cancers cells after treatment with ASBDs was linked to the reduced activity of kinases Akt, Erk1/2 and p38 and the induction of apoptosis, as investigated using Western blotting and FACS. In addition, in silico analyses of the ADMET profile found EB5 to be a promising anti-cancer drug candidate that would benefit from further investigation.
Keywords: ADMET; acetylenic synthetic betulin derivatives; betulin; chemotherapy; cisplatin; druglikeness; neuroblastoma; pediatric cancers; rhabdomyosarcoma; temozolomide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





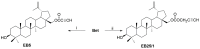
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

