Intrauterine L-NAME Exposure Weakens the Development of Sympathetic Innervation and Induces the Remodeling of Arterial Vessels in Two-Week-Old Rats
- PMID: 34830206
- PMCID: PMC8618620
- DOI: 10.3390/ijms222212327
Intrauterine L-NAME Exposure Weakens the Development of Sympathetic Innervation and Induces the Remodeling of Arterial Vessels in Two-Week-Old Rats
Abstract
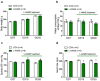
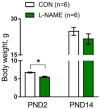
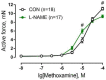
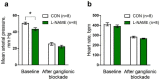
Nitric oxide (NO) has been shown to stimulate differentiation and increase the survival of ganglionic sympathetic neurons. The proportion of neuronal NOS-immunoreactive sympathetic preganglionic neurons is particularly high in newborn rats and decreases with maturation. However, the role of NO in the development of vascular sympathetic innervation has never been studied before. We tested the hypothesis that intrauterine NO deficiency weakened the development of vascular sympathetic innervation and thereby changed the contractility of peripheral arteries and blood pressure level in two-week-old offspring. Pregnant rats consumed NOS inhibitor L-NAME (250 mg/L in drinking water) from gestational day 10 until delivery. Pups in the L-NAME group had a reduced body weight and blood level of NO metabolites at 1-2 postnatal days. Saphenous arteries from two-week-old L-NAME offspring demonstrated a lower density of sympathetic innervation, a smaller inner diameter, reduced maximal active force and decreased α-actin/β-actin mRNA expression ratio compared to the controls. Importantly, pups in the L-NAME group exhibited decreased blood pressure levels before, but not after, ganglionic blockade with chlorisondamine. In conclusion, intrauterine L-NAME exposure is followed by the impaired development of the sympathetic nervous system in early postnatal life, which is accompanied by the structural and functional remodeling of arterial blood vessels.
Keywords: blood pressure; early postnatal development; nitric oxide; sympathetic innervation; vasculature.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Endothelial nitric oxide weakens arterial contractile responses and reduces blood pressure during early postnatal development in rats.Nitric Oxide. 2016 May 1;55-56:1-9. doi: 10.1016/j.niox.2016.02.005. Epub 2016 Feb 23. Nitric Oxide. 2016. PMID: 26923819
-
Effect of inhibition of nitric oxide synthase on blood pressure and renal sodium handling in renal denervated rats.Braz J Med Biol Res. 2000 Mar;33(3):347-54. doi: 10.1590/s0100-879x2000000300014. Braz J Med Biol Res. 2000. PMID: 10719388
-
Role of prostaglandin, endothelin and sympathetic nervous system on the L-NAME-induced pressor responses in spontaneously hypertensive rats.Brain Res. 2003 Sep 5;983(1-2):162-73. doi: 10.1016/s0006-8993(03)03052-x. Brain Res. 2003. PMID: 12914977
-
Behavioural, neurochemical and neuroanatomical effects of chronic postnatal N-nitro-L-arginine methyl ester treatment in neonatal and adult rats.Neuroscience. 1998 Nov;87(1):181-95. doi: 10.1016/s0306-4522(98)00083-9. Neuroscience. 1998. PMID: 9722151
-
[The functions of arterial sympathetic innervation: from development to pathology].Med Sci (Paris). 2019 Aug-Sep;35(8-9):643-650. doi: 10.1051/medsci/2019131. Epub 2019 Sep 18. Med Sci (Paris). 2019. PMID: 31532376 Review. French.
Cited by
-
Regulation and Pharmacology of the Cyclic GMP and Nitric Oxide Pathway in Embryonic and Adult Stem Cells.Cells. 2024 Dec 5;13(23):2008. doi: 10.3390/cells13232008. Cells. 2024. PMID: 39682756 Free PMC article. Review.
-
Reliability of Rodent and Rabbit Models in Preeclampsia Research.Int J Mol Sci. 2022 Nov 18;23(22):14344. doi: 10.3390/ijms232214344. Int J Mol Sci. 2022. PMID: 36430816 Free PMC article. Review.
References
-
- Sofronova S.I., Borzykh A.A., Gaynullina D.K., Kuzmin I.V., Shvetsova A.A., Lukoshkova E.V., Tarasova O.S. Endothelial nitric oxide weakens arterial contractile responses and reduces blood pressure during early postnatal development in rats. Nitric Oxide. 2016;55–56:1–9. doi: 10.1016/j.niox.2016.02.005. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

