NGAL as a Potential Target in Tumor Microenvironment
- PMID: 34830212
- PMCID: PMC8623964
- DOI: 10.3390/ijms222212333
NGAL as a Potential Target in Tumor Microenvironment
Abstract
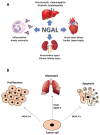
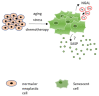
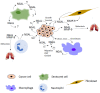
The signaling network between cancer and stromal cells plays a crucial role in tumor microenvironment. The fate of tumor progression mainly depends on the huge amount of information that these cell populations exchange from the onset of neoplastic transformation. Interfering with such signaling has been producing exciting results in cancer therapy: just think of anti-PD-1/anti-PD-L1/anti-CTLA-4 antibodies that, acting as immune checkpoint inhibitors, interrupt the inhibitory signaling exerted by cancer cells on immune cells or the CAR-T technology that fosters the reactivation of anti-tumoral immunity in a restricted group of leukemias and lymphomas. Nevertheless, many types of cancers, in particular solid tumors, are still refractory to these treatments, so the identification of novel molecular targets in tumor secretome would benefit from implementation of current anti-cancer therapeutical strategies. Neutrophil Gelatinase-Associated Lipocalin (NGAL) is a secreted protein abundantly expressed in the secretome of various human tumors. It represents a promising target for the multiple roles that are played inside cancer and stromal cells, and also overall in their cross-talk. The review focuses on the different roles of NGAL in tumor microenvironment and in cancer senescence-associated secretory phenotype (SASP), highlighting the most crucial functions that could be eventually targetable in cancer therapy.
Keywords: NGAL; SASP; iron; siderophores; tumor stroma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Winck F.V., Prado Ribeiro A.C., Ramos Domingues R., Ling L.Y., Riano-Pachon D.M., Rivera C., Brandão T.B., Gouvea A.F., Santos-Silva A.R., Coletta R.D., et al. Insights into immune responses in oral cancer through proteomic analysis of saliva and salivary extracellular vesicles. Sci. Rep. 2015;5:16305. doi: 10.1038/srep16305. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

