NTRK Gene Fusion Detection in Atypical Spitz Tumors
- PMID: 34830218
- PMCID: PMC8619835
- DOI: 10.3390/ijms222212332
NTRK Gene Fusion Detection in Atypical Spitz Tumors
Abstract
Atypical Spitz tumors (AST) deviate from stereotypical Spitz nevi for one or more atypical features and are now regarded as an intermediate category of melanocytic tumors with uncertain malignant potential. Activating NTRK1/NTRK3 fusions elicit oncogenic events in Spitz lesions and are targetable with kinase inhibitors. However, their prevalence among ASTs and the optimal approach for their detection is yet to be determined. A series of 180 ASTs were screened with pan-TRK immunohistochemistry and the presence of NTRK fusions was confirmed using FISH, two different RNA-based NGS panels for solid tumors, and a specific real time RT-PCR panel. Overall, 26 ASTs showed pan-TRK immunostaining. NTRK1 fusions were detected in 15 of these cases showing cytoplasmic immunoreaction, whereas NTRK3 was detected in one case showing nuclear immunoreaction. Molecular tests resulted all positive in only two ASTs (included the NTRK3 translocated), RNA-based NGS and real time RT-PCR were both positive in three cases, and FISH and real time RT-PCR in another two cases. In seven ASTs NTRK1 fusions were detected only by FISH and in two cases only by real time RT-PCR. The frequency of NTRK fusions in ASTs is 9%, with a clear prevalence of NTRK1 compared to NTRK3 alterations. Pan-TRK immunohistochemistry is an excellent screening test. Confirmation of NTRK fusions may require the use of different molecular techniques.
Keywords: NTRK; NTRK1; NTRK3; atypical spitz tumor; pan-TRK.
Conflict of interest statement
DM has received honoraria for professional services and consultancy for Bayer HealthCare Pharmaceuticals Inc. (Whippany, NJ, USA). The remaining authors declare that they have no conflict of interest.
Figures


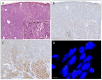
References
-
- Gerami P., Busam K., Cochran A., Cook M.G., Duncan L.M., Elder D.E., Fullen D.R., Guitart J., LeBoit P.E., Mihm M.C., Jr., et al. Histomorphologic assessment and interobserver diagnostic reproducibility of atypical spitzoid melanocytic neoplasms with long-term follow-up. Am. J. Surg. Pathol. 2014;38:934–940. doi: 10.1097/PAS.0000000000000198. - DOI - PubMed
-
- Cerroni L., Barnhill R., Elder D., Gottlieb G., Heenan P., Kutzner H., LeBoit P.E., Mihm M., Jr., Rosai J., Kerl H. Melanocytic tumors of uncertain malignant potential: Results of a tutorial held at the XXIX Symposium of the International Society of Dermatopathology in Graz, October 2008. Am. J. Surg. Pathol. 2010;34:314–326. doi: 10.1097/PAS.0b013e3181cf7fa0. - DOI - PubMed

