Exercise Training Alleviates Cardiac Fibrosis through Increasing Fibroblast Growth Factor 21 and Regulating TGF-β1-Smad2/3-MMP2/9 Signaling in Mice with Myocardial Infarction
- PMID: 34830222
- PMCID: PMC8623999
- DOI: 10.3390/ijms222212341
Exercise Training Alleviates Cardiac Fibrosis through Increasing Fibroblast Growth Factor 21 and Regulating TGF-β1-Smad2/3-MMP2/9 Signaling in Mice with Myocardial Infarction
Abstract
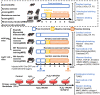
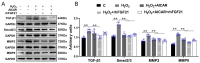
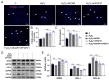
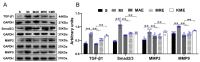
Exercise training has been reported to alleviate cardiac fibrosis and ameliorate heart dysfunction after myocardial infarction (MI), but the molecular mechanism is still not fully clarified. Fibroblast growth factor 21 (FGF21) exerts a protective effect on the infarcted heart. This study investigates whether exercise training could increase FGF21 protein expression and regulate the transforming growth factor-β1 (TGF-β1)-Smad2/3-MMP2/9 signaling pathway to alleviate cardiac fibrosis following MI. Male wild type (WT) C57BL/6J mice and Fgf21 knockout (Fgf21 KO) mice were used to establish the MI model and subjected to five weeks of different types of exercise training. Both aerobic exercise training (AET) and resistance exercise training (RET) significantly alleviated cardiac dysfunction and fibrosis, up-regulated FGF21 protein expression, inhibited the activation of TGF-β1-Smad2/3-MMP2/9 signaling pathway and collagen production, and meanwhile, enhanced antioxidant capacity and reduced cell apoptosis in the infarcted heart. In contrast, knockout of Fgf21 weakened the cardioprotective effects of AET after MI. In vitro, cardiac fibroblasts (CFs) were isolated from neonatal mice hearts and treated with H2O2 (100 μM, 6 h). Recombinant human FGF21 (rhFGF21, 100 ng/mL, 15 h) and/or 5-Aminoimidazole-4-carboxamide ribonucleotide (AICAR, 1 mM, 15 h) inhibited H2O2-induced activation of the TGF-β1-Smad2/3-MMP2/9 signaling pathway, promoted CFs apoptosis and reduced collagen production. In conclusion, exercise training increases FGF21 protein expression, inactivates the TGF-β1-Smad2/3-MMP2/9 signaling pathway, alleviates cardiac fibrosis, oxidative stress, and cell apoptosis, and finally improves cardiac function in mice with MI. FGF21 plays an important role in the anti-fibrosis effect of exercise training.
Keywords: cardiac fibrosis; exercise training; fibroblast growth factor 21; myocardial infarction.
Conflict of interest statement
All the authors declare that there are no conflict of interest in this article.
Figures



Similar articles
-
Notch3 Ameliorates Cardiac Fibrosis After Myocardial Infarction by Inhibiting the TGF-β1/Smad3 Pathway.Cardiovasc Toxicol. 2016 Oct;16(4):316-24. doi: 10.1007/s12012-015-9341-z. Cardiovasc Toxicol. 2016. PMID: 26487518
-
Resistance exercise upregulates Irisin expression and suppresses myocardial fibrosis following myocardial infarction via activating AMPK-Sirt1 and inactivating TGFβ1-Smad2/3.Acta Physiol (Oxf). 2024 Jul;240(7):e14163. doi: 10.1111/apha.14163. Epub 2024 May 16. Acta Physiol (Oxf). 2024. PMID: 38752665
-
Concurrent vitamin D supplementation and exercise training improve cardiac fibrosis via TGF-β/Smad signaling in myocardial infarction model of rats.J Physiol Biochem. 2021 Feb;77(1):75-84. doi: 10.1007/s13105-020-00778-6. Epub 2021 Jan 11. J Physiol Biochem. 2021. PMID: 33428175
-
The role of Smad signaling cascades in cardiac fibrosis.Cell Signal. 2021 Jan;77:109826. doi: 10.1016/j.cellsig.2020.109826. Epub 2020 Nov 5. Cell Signal. 2021. PMID: 33160018 Free PMC article. Review.
-
Fibroblast contributions to ischemic cardiac remodeling.Cell Signal. 2021 Jan;77:109824. doi: 10.1016/j.cellsig.2020.109824. Epub 2020 Nov 2. Cell Signal. 2021. PMID: 33144186 Free PMC article. Review.
Cited by
-
Aerobic Exercise Ameliorates Myocardial Fibrosis via Affecting Vitamin D Receptor and Transforming Growth Factor-β1 Signaling in Vitamin D-Deficient Mice.Nutrients. 2023 Feb 1;15(3):741. doi: 10.3390/nu15030741. Nutrients. 2023. PMID: 36771445 Free PMC article.
-
Activation of Cholinergic Anti-Inflammatory Pathway Ameliorates Cerebral and Cardiac Dysfunction After Intracerebral Hemorrhage Through Autophagy.Front Immunol. 2022 Jun 23;13:870174. doi: 10.3389/fimmu.2022.870174. eCollection 2022. Front Immunol. 2022. PMID: 35812436 Free PMC article.
-
Whole-body vibration as a passive alternative to exercise after myocardial damage in middle-aged female rats: Effects on the heart, the brain, and behavior.Front Aging Neurosci. 2023 Mar 7;15:1034474. doi: 10.3389/fnagi.2023.1034474. eCollection 2023. Front Aging Neurosci. 2023. PMID: 36960421 Free PMC article.
-
Low-Intensity Physical Exercise Decreases Inflammation and Joint Damage in the Preclinical Phase of a Rheumatoid Arthritis Murine Model.Biomolecules. 2023 Mar 7;13(3):488. doi: 10.3390/biom13030488. Biomolecules. 2023. PMID: 36979423 Free PMC article.
-
Circulating fibroblast growth factor-21, Omentin-1, and betatrophin and their association with metabolic parameters in Saudi adolescents post 12-month lifestyle change program.BMC Pediatr. 2025 Jul 1;25(1):482. doi: 10.1186/s12887-025-05833-z. BMC Pediatr. 2025. PMID: 40597046 Free PMC article.
References
-
- Virani S.S., Alonso A., Aparicio H.J., Benjamin E.J., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Cheng S., Delling F.N., et al. Heart Disease and Stroke Statistics-2021 Update: A Report from the American Heart Association. Circulation. 2021;143:e254–e743. doi: 10.1161/CIR.0000000000000950. - DOI - PubMed
-
- Roth G.A., Mensah G.A., Johnson C.O., Addolorato G., Ammirati E., Baddour L.M., Barengo N.C., Beaton A.Z., Benjamin E.J., Benziger C.P., et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010. - DOI - PMC - PubMed
-
- Li H.L., Zhuo M.L., Wang D., Wang A.B., Cai H., Sun L.H., Yang Q., Huang Y., Wei Y.S., Liu P.P., et al. Targeted cardiac overexpression of A20 improves left ventricular performance and reduces compensatory hypertrophy after myocardial infarction. Circulation. 2007;115:1885–1894. doi: 10.1161/CIRCULATIONAHA.106.656835. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

