Albumin-Hyaluronan Interactions: Influence of Ionic Composition Probed by Molecular Dynamics
- PMID: 34830249
- PMCID: PMC8625520
- DOI: 10.3390/ijms222212360
Albumin-Hyaluronan Interactions: Influence of Ionic Composition Probed by Molecular Dynamics
Abstract
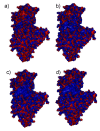
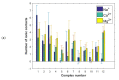
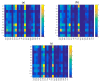
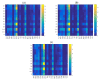
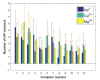
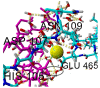
The lubrication mechanism in synovial fluid and joints is not yet fully understood. Nevertheless, intermolecular interactions between various neutral and ionic species including large macromolecular systems and simple inorganic ions are the key to understanding the excellent lubrication performance. An important tool for characterizing the intermolecular forces and their structural consequences is molecular dynamics. Albumin is one of the major components in synovial fluid. Its electrostatic properties, including the ability to form molecular complexes, are closely related to pH, solvation, and the presence of ions. In the context of synovial fluid, it is relevant to describe the possible interactions between albumin and hyaluronate, taking into account solution composition effects. In this study, the influence of Na+, Mg2+, and Ca2+ ions on human serum albumin-hyaluronan interactions were examined using molecular dynamics tools. It was established that the presence of divalent cations, and especially Ca2+, contributes mostly to the increase of the affinity between hyaluronan and albumin, which is associated with charge compensation in negatively charged hyaluronan and albumin. Furthermore, the most probable binding sites were structurally and energetically characterized. The indicated moieties exhibit a locally positive charge which enables hyaluronate binding (direct and water mediated).
Keywords: human serum albumin; hyaluronan; hyaluronic acid; hydrogen bonds; ionic interactions; molecular dynamics simulations; water mediated interactions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

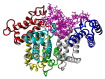





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

