Modeling, Synthesis, and Biological Evaluation of Potential Retinoid-X-Receptor (RXR) Selective Agonists: Analogs of 4-[1-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahyro-2-naphthyl)ethynyl]benzoic Acid (Bexarotene) and 6-(Ethyl(4-isobutoxy-3-isopropylphenyl)amino)nicotinic Acid (NEt-4IB)
- PMID: 34830251
- PMCID: PMC8624485
- DOI: 10.3390/ijms222212371
Modeling, Synthesis, and Biological Evaluation of Potential Retinoid-X-Receptor (RXR) Selective Agonists: Analogs of 4-[1-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahyro-2-naphthyl)ethynyl]benzoic Acid (Bexarotene) and 6-(Ethyl(4-isobutoxy-3-isopropylphenyl)amino)nicotinic Acid (NEt-4IB)
Abstract
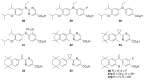
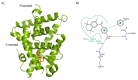
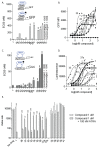
Five novel analogs of 6-(ethyl)(4-isobutoxy-3-isopropylphenyl)amino)nicotinic acid-or NEt-4IB-in addition to seven novel analogs of 4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydro-2-naphthyl)ethynyl]benzoic acid (bexarotene) were prepared and evaluated for selective retinoid-X-receptor (RXR) agonism alongside bexarotene (1), a FDA-approved drug for cutaneous T-cell lymphoma (CTCL). Bexarotene treatment elicits side-effects by provoking or disrupting other RXR-dependent pathways. Analogs were assessed by the modeling of binding to RXR and then evaluated in a human cell-based RXR-RXR mammalian-2-hybrid (M2H) system as well as a RXRE-controlled transcriptional system. The analogs were also tested in KMT2A-MLLT3 leukemia cells and the EC50 and IC50 values were determined for these compounds. Moreover, the analogs were assessed for activation of LXR in an LXRE system as drivers of ApoE expression and subsequent use as potential therapeutics in neurodegenerative disorders, and the results revealed that these compounds exerted a range of differential LXR-RXR activation and selectivity. Furthermore, several of the novel analogs in this study exhibited reduced RARE cross-signaling, implying RXR selectivity. These results demonstrate that modification of partial agonists such as NEt-4IB and potent rexinoids such as bexarotene can lead to compounds with improved RXR selectivity, decreased cross-signaling of other RXR-dependent nuclear receptors, increased LXRE-heterodimer selectivity, and enhanced anti-proliferative potential in leukemia cell lines compared to therapeutics such as 1.
Keywords: leukemia; retinoid; retinoid-X-receptor; rexinoid; small molecule therapeutic; structure–activity relationship.
Conflict of interest statement
The authors declare no conflict of interest. Patent applications covering the technologies described in this work have been applied for on behalf of the Arizona Board of Regents.
Figures






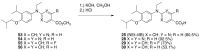
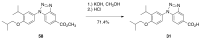














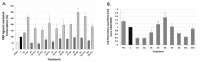

Similar articles
-
An Isochroman Analog of CD3254 and Allyl-, Isochroman-Analogs of NEt-TMN Prove to Be More Potent Retinoid-X-Receptor (RXR) Selective Agonists Than Bexarotene.Int J Mol Sci. 2022 Dec 19;23(24):16213. doi: 10.3390/ijms232416213. Int J Mol Sci. 2022. PMID: 36555852 Free PMC article.
-
Modeling, synthesis and cell-based evaluation of pyridine-substituted analogs of CD3254 and fluorinated analogs of CBt-PMN as novel therapeutics.Bioorg Med Chem. 2025 Mar 1;119:118059. doi: 10.1016/j.bmc.2024.118059. Epub 2025 Jan 2. Bioorg Med Chem. 2025. PMID: 39808894
-
Modeling, Synthesis, and Biological Evaluation of Potential Retinoid X Receptor (RXR)-Selective Agonists: Analogues of 4-[1-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro-2-naphthyl)ethynyl]benzoic Acid (Bexarotene) and 6-(Ethyl(5,5,8,8-tetrahydronaphthalen-2-yl)amino)nicotinic Acid (NEt-TMN).J Med Chem. 2016 Oct 13;59(19):8924-8940. doi: 10.1021/acs.jmedchem.6b00812. Epub 2016 Sep 19. J Med Chem. 2016. PMID: 27592633
-
Retinoid X Receptor agonists as selective modulators of the immune system for the treatment of cancer.Pharmacol Ther. 2023 Dec;252:108561. doi: 10.1016/j.pharmthera.2023.108561. Epub 2023 Nov 10. Pharmacol Ther. 2023. PMID: 37952906 Free PMC article. Review.
-
Potential therapeutic uses of rexinoids.Adv Pharmacol. 2021;91:141-183. doi: 10.1016/bs.apha.2021.01.004. Epub 2021 Mar 4. Adv Pharmacol. 2021. PMID: 34099107 Review.
Cited by
-
An Isochroman Analog of CD3254 and Allyl-, Isochroman-Analogs of NEt-TMN Prove to Be More Potent Retinoid-X-Receptor (RXR) Selective Agonists Than Bexarotene.Int J Mol Sci. 2022 Dec 19;23(24):16213. doi: 10.3390/ijms232416213. Int J Mol Sci. 2022. PMID: 36555852 Free PMC article.
-
BRF110, an Orally Active Nurr1-RXRα-Selective Rexinoid, Enhances BDNF Expression without Elevating Triglycerides.J Med Chem. 2025 Feb 27;68(4):4763-4786. doi: 10.1021/acs.jmedchem.4c03046. Epub 2025 Feb 13. J Med Chem. 2025. PMID: 39945195 Free PMC article.
-
Role of Nuclear Receptors on the Progression of Multiple Sclerosis: A Review.Cell Mol Neurobiol. 2025 Jun 17;45(1):58. doi: 10.1007/s10571-025-01563-z. Cell Mol Neurobiol. 2025. PMID: 40526306 Free PMC article. Review.
-
Synergistic Activation of VDR-RXR Heterodimers by Vitamin D and Rexinoids in Human Kidney and Brain Cells.Cells. 2024 Nov 14;13(22):1878. doi: 10.3390/cells13221878. Cells. 2024. PMID: 39594626 Free PMC article.
-
Development of Bexarotene Analogs for Treating Cutaneous T-Cell Lymphomas.Cells. 2023 Nov 4;12(21):2575. doi: 10.3390/cells12212575. Cells. 2023. PMID: 37947652 Free PMC article.
References
-
- Mangelsdorf D.J.U., Evans R.M.K. The Retinoids. Academic Press; Orlando, FL, USA: 1994. pp. 319–349.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

