COX Inhibitory and Cytotoxic Naphthoketal-Bearing Polyketides from Sparticola junci
- PMID: 34830260
- PMCID: PMC8619024
- DOI: 10.3390/ijms222212379
COX Inhibitory and Cytotoxic Naphthoketal-Bearing Polyketides from Sparticola junci
Abstract
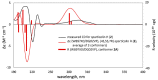
Axenic fermentation on solid rice of the saprobic fungus Sparticola junci afforded two new highly oxidized naphthalenoid polyketide derivatives, sparticatechol A (1) and sparticolin H (2) along with sparticolin A (3). The structures of 1 and 2 were elucidated on the basis of their NMR and HR-ESIMS spectroscopic data. Assignment of absolute configurations was performed using electronic circular dichroism (ECD) experiments and Time-Dependent Density Functional Theory (TDDFT) calculations. Compounds 1-3 were evaluated for COX inhibitory, antiproliferative, cytotoxic and antimicrobial activities. Compounds 1 and 2 exhibited strong inhibitory activities against COX-1 and COX-2. Molecular docking analysis of 1 conferred favorable binding against COX-2. Sparticolin H (2) and A (3) showed a moderate antiproliferative effect against myelogenous leukemia K-562 cells and weak cytotoxicity against HeLa and mouse fibroblast cells.
Keywords: COX inhibitory; ECD-TDDFT; Sparticola junci; antiproliferative; cytotoxic; molecular docking; structure elucidation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Catechol-Bearing Polyketide Derivatives from Sparticola junci.J Nat Prod. 2021 Jul 23;84(7):2053-2058. doi: 10.1021/acs.jnatprod.1c00415. Epub 2021 Jul 1. J Nat Prod. 2021. PMID: 34197704
-
Cytospyrone and Cytospomarin: Two New Polyketides Isolated from Mangrove Endophytic Fungus, Cytospora sp.Molecules. 2020 Sep 15;25(18):4224. doi: 10.3390/molecules25184224. Molecules. 2020. PMID: 32942587 Free PMC article.
-
Cladosins L-O, new hybrid polyketides from the endophytic fungus Cladosporium sphaerospermum WBS017.Eur J Med Chem. 2020 Apr 1;191:112159. doi: 10.1016/j.ejmech.2020.112159. Epub 2020 Feb 19. Eur J Med Chem. 2020. PMID: 32101782
-
Cytotoxic Polyketides from the Marine Sponge-Derived Fungus Pestalotiopsis heterocornis XWS03F09.Molecules. 2019 Jul 22;24(14):2655. doi: 10.3390/molecules24142655. Molecules. 2019. PMID: 31336683 Free PMC article.
-
Ulleungdolin, a Polyketide-Peptide Hybrid Bearing a 2,4-Di-O-methyl-β-d-antiarose from Streptomyces sp. 13F051 Co-cultured with Leohumicola minima 15S071.J Nat Prod. 2022 Oct 28;85(10):2445-2453. doi: 10.1021/acs.jnatprod.2c00682. Epub 2022 Oct 5. J Nat Prod. 2022. PMID: 36197044 Review.
Cited by
-
In silico and ADMET molecular analysis targeted to discover novel anti-inflammatory drug candidates as COX-2 inhibitors from specific metabolites of Diospyros batokana (Ebenaceae).Biochem Biophys Rep. 2024 Jul 13;39:101758. doi: 10.1016/j.bbrep.2024.101758. eCollection 2024 Sep. Biochem Biophys Rep. 2024. PMID: 39108619 Free PMC article.
-
Adipose tissue as target of environmental toxicants: focus on mitochondrial dysfunction and oxidative inflammation in metabolic dysfunction-associated steatotic liver disease.Mol Cell Biochem. 2025 May;480(5):2863-2879. doi: 10.1007/s11010-024-05165-z. Epub 2024 Dec 20. Mol Cell Biochem. 2025. PMID: 39704874 Free PMC article. Review.
-
Polyoxygenated Cyclohexenes from Uvaria grandiflora with Multi-Enzyme Targeting Properties Relevant in Type 2 Diabetes and Obesity.ACS Omega. 2022 Oct 7;7(41):36856-36864. doi: 10.1021/acsomega.2c05544. eCollection 2022 Oct 18. ACS Omega. 2022. PMID: 36278100 Free PMC article.
-
Attenuation of Lipopolysaccharide-Induced Inflammatory Responses through Inhibition of the NF-κB Pathway and the Increased NRF2 Level by a Flavonol-Enriched n-Butanol Fraction from Uvaria alba.ACS Omega. 2023 Jan 31;8(6):5377-5392. doi: 10.1021/acsomega.2c06451. eCollection 2023 Feb 14. ACS Omega. 2023. PMID: 36816691 Free PMC article.
-
Editorial to Special Issue "Theme Issue Honoring Prof. Dr. Ludger Wessjohann's 60th Birthday: Natural Products in Modern Drug Discovery".Int J Mol Sci. 2022 May 23;23(10):5835. doi: 10.3390/ijms23105835. Int J Mol Sci. 2022. PMID: 35628644 Free PMC article.
References
-
- Kuephadungphan W., Macabeo A.P.G., Luangsa-ard J.J., Stadler M. Discovery of novel biologically active secondary metabolites from Thai mycodiversity with anti-infective potential. CRBIOT. 2004;3:160–172. doi: 10.1016/j.crbiot.2021.05.003. - DOI
-
- Hyde K.D., Xu J., Rapior S., Jeewon R., Lumyong S., Niego A.G.T., Abeywickrama P.D., Aluthmuhandiram J.V.S., Brahamanage R.S., Brooks S., et al. The amazing potential of fungi: 50 ways we can exploit fungi industrially. Fungal Divers. 2019;97:1–136.
-
- Ogishi H., Chiba N., Mikawa T., Sasaki T., Miyaji S., Sezaki M. Mitsubishi Kasei Corp., JP 01294686. Chem. Abstr. 1990;113:38906q.
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

