Ultraviolet Treatment of Titanium to Enhance Adhesion and Retention of Oral Mucosa Connective Tissue and Fibroblasts
- PMID: 34830275
- PMCID: PMC8617952
- DOI: 10.3390/ijms222212396
Ultraviolet Treatment of Titanium to Enhance Adhesion and Retention of Oral Mucosa Connective Tissue and Fibroblasts
Abstract
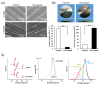
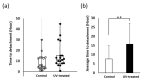
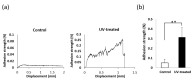
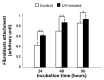
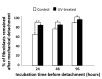
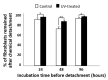
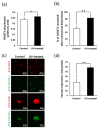
Peri-implantitis is an unsolved but critical problem with dental implants. It is postulated that creating a seal of gingival soft tissue around the implant neck is key to preventing peri-implantitis. The objective of this study was to determine the effect of UV surface treatment of titanium disks on the adhesion strength and retention time of oral connective tissues as well as on the adherence of mucosal fibroblasts. Titanium disks with a smooth machined surface were prepared and treated with UV light for 15 min. Keratinized mucosal tissue sections (3 × 3 mm) from rat palates were incubated for 24 h on the titanium disks. The adhered tissue sections were then mechanically detached by agitating the culture dishes. The tissue sections remained adherent for significantly longer (15.5 h) on the UV-treated disks than on the untreated control disks (7.5 h). A total of 94% of the tissue sections were adherent for 5 h or longer on the UV-treated disks, whereas only 50% of the sections remained on the control disks for 5 h. The adhesion strength of the tissue sections to the titanium disks, as measured by tensile testing, was six times greater after UV treatment. In the culture studies, mucosal fibroblasts extracted from rat palates were attached to titanium disks by incubating for 24, 48, or 96 h. The number of attached cells was consistently 15-30% greater on the UV-treated disks than on the control disks. The cells were then subjected to mechanical or chemical (trypsinization) detachment. After mechanical detachment, the residual cell rates on the UV-treated surfaces after 24 and 48 h of incubation were 35% and 25% higher, respectively, than those on the control surfaces. The remaining rate after chemical detachment was 74% on the control surface and 88% on the UV-treated surface for the cells cultured for 48 h. These trends were also confirmed in mouse embryonic fibroblasts, with an intense expression of vinculin, a focal adhesion protein, on the UV-treated disks even after detachment. The UV-treated titanium was superhydrophilic, whereas the control titanium was hydrophobic. X-ray photoelectron spectroscopy (XPS) chemical analysis revealed that the amount of carbon at the surface was significantly reduced after UV treatment, while the amount of TiOH molecules was increased. These ex vivo and in vitro results indicate that the UV treatment of titanium increases the adhesion and retention of oral mucosa connective tissue as a result of increased resistance of constituent fibroblasts against exogenous detachment, both mechanically and chemically, as well as UV-induced physicochemical changes of the titanium surface.
Keywords: UV treatment; connective tissue; fibroblast; surface characteristics; titanium implant.
Conflict of interest statement
The authors declare no conflict of interest. The sponsors had no role in the design, execution, interpretation, or writing of the study.
Figures

Similar articles
-
Enhancement of osteoblast adhesion to UV-photofunctionalized titanium via an electrostatic mechanism.Biomaterials. 2010 Apr;31(10):2717-27. doi: 10.1016/j.biomaterials.2009.12.024. Epub 2009 Dec 24. Biomaterials. 2010. PMID: 20035996
-
The enhanced characteristics of osteoblast adhesion to photofunctionalized nanoscale TiO2 layers on biomaterials surfaces.Biomaterials. 2010 May;31(14):3827-39. doi: 10.1016/j.biomaterials.2010.01.133. Epub 2010 Feb 13. Biomaterials. 2010. PMID: 20153521
-
Enhancement of adhesion strength and cellular stiffness of osteoblasts on mirror-polished titanium surface by UV-photofunctionalization.Acta Biomater. 2010 Dec;6(12):4578-88. doi: 10.1016/j.actbio.2010.07.010. Epub 2010 Jul 13. Acta Biomater. 2010. PMID: 20633705
-
Ultraviolet photofunctionalization of titanium implants.Int J Oral Maxillofac Implants. 2014 Jan-Feb;29(1):e95-102. doi: 10.11607/jomi.te47. Int J Oral Maxillofac Implants. 2014. PMID: 24451893 Review.
-
Does Ultraviolet Radiation Exhibit Antimicrobial Effect against Oral Pathogens Attached on Various Dental Implant Surfaces? A Systematic Review.Dent J (Basel). 2022 May 31;10(6):93. doi: 10.3390/dj10060093. Dent J (Basel). 2022. PMID: 35735635 Free PMC article. Review.
Cited by
-
Surface modification strategies to reinforce the soft tissue seal at transmucosal region of dental implants.Bioact Mater. 2024 Sep 10;42:404-432. doi: 10.1016/j.bioactmat.2024.08.042. eCollection 2024 Dec. Bioact Mater. 2024. PMID: 39308548 Free PMC article. Review.
-
Human Gingival Fibroblast Attachment to Smooth Titanium Disks with Different Surface Roughnesses.Biomimetics (Basel). 2022 Oct 14;7(4):164. doi: 10.3390/biomimetics7040164. Biomimetics (Basel). 2022. PMID: 36278721 Free PMC article.
-
Promoted Abutment-Soft Tissue Integration Around Self-Glazed Zirconia Surfaces with Nanotopography Fabricated by Additive 3D Gel Deposition.Int J Nanomedicine. 2023 Jun 13;18:3141-3155. doi: 10.2147/IJN.S404047. eCollection 2023. Int J Nanomedicine. 2023. PMID: 37333732 Free PMC article.
-
Graphene oxide and mineralized collagen-functionalized dental implant abutment with effective soft tissue seal and romotely repeatable photodisinfection.Regen Biomater. 2022 Apr 29;9:rbac024. doi: 10.1093/rb/rbac024. eCollection 2022. Regen Biomater. 2022. PMID: 35529047 Free PMC article.
-
Decomposing Organic Molecules on Titanium with Vacuum Ultraviolet Light for Effective and Rapid Photofunctionalization.J Funct Biomater. 2022 Dec 23;14(1):11. doi: 10.3390/jfb14010011. J Funct Biomater. 2022. PMID: 36662058 Free PMC article.
References
-
- Saruwatari L., Aita H., Butz F., Nakamura H.K., Ouyang J., Yang Y., Chiou W.-A., Ogawa T. Osteoblasts generate harder, stiffer, and more delamination-resistant mineralized tissue on titanium than on polystyrene, associated with distinct tissue micro- and ultrastructure. J. Bone Miner. Res. 2005;20:2002–2016. doi: 10.1359/JBMR.050703. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

