Keratins as an Inflammation Trigger Point in Epidermolysis Bullosa Simplex
- PMID: 34830328
- PMCID: PMC8624175
- DOI: 10.3390/ijms222212446
Keratins as an Inflammation Trigger Point in Epidermolysis Bullosa Simplex
Abstract
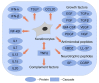
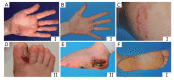
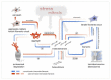
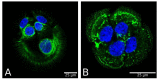
Epidermolysis bullosa simplex (EBS) is a group of inherited keratinopathies that, in most cases, arise due to mutations in keratins and lead to intraepidermal ruptures. The cellular pathology of most EBS subtypes is associated with the fragility of the intermediate filament network, cytolysis of the basal layer of the epidermis, or attenuation of hemidesmosomal/desmosomal components. Mutations in keratins 5/14 or in other genes that encode associated proteins induce structural disarrangements of different strengths depending on their locations in the genes. Keratin aggregates display impaired dynamics of assembly and diminished solubility and appear to be the trigger for endoplasmic reticulum (ER) stress upon being phosphorylated by MAPKs. Global changes in cellular signaling mainly occur in cases of severe dominant EBS mutations. The spectrum of changes initiated by phosphorylation includes the inhibition of proteasome degradation, TNF-α signaling activation, deregulated proliferation, abnormal cell migration, and impaired adherence of keratinocytes. ER stress also leads to the release of proinflammatory danger-associated molecular pattern (DAMP) molecules, which enhance avalanche-like inflammation. Many instances of positive feedback in the course of cellular stress and the development of sterile inflammation led to systemic chronic inflammation in EBS. This highlights the role of keratin in the maintenance of epidermal and immune homeostasis.
Keywords: aggregation; basal layer; blistering; chemokine; cytokine; epidermis; epidermolysis bullosa simplex; inflammation; injury; keratin; keratinocyte; mutation; phosphorylation; proinflammatory cascade; skin; stress; wound healing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Morley S.M., D’Alessandro M., Sexton C., Rugg E.L., Navsaria H., Shemanko C.S., Huber M., Hohl D., Heagerty A.I., Leigh I.M., et al. Generation and Characterization of Epidermolysis Bullosa Simplex Cell Lines: Scratch Assays Show Faster Migration with Disruptive Keratin Mutations. Br. J. Dermatol. 2003;149:46–58. doi: 10.1046/j.1365-2133.2003.05493.x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

