Proteomic Analysis of Marinesco-Sjogren Syndrome Fibroblasts Indicates Pro-Survival Metabolic Adaptation to SIL1 Loss
- PMID: 34830330
- PMCID: PMC8620507
- DOI: 10.3390/ijms222212449
Proteomic Analysis of Marinesco-Sjogren Syndrome Fibroblasts Indicates Pro-Survival Metabolic Adaptation to SIL1 Loss
Abstract
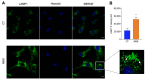
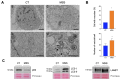
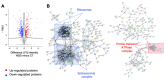
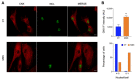
Marinesco-Sjogren syndrome (MSS) is a rare multisystem pediatric disorder, caused by loss-of-function mutations in the gene encoding the endoplasmic reticulum cochaperone SIL1. SIL1 acts as a nucleotide exchange factor for BiP, which plays a central role in secretory protein folding. SIL1 mutant cells have reduced BiP-assisted protein folding, cannot fulfil their protein needs, and experience chronic activation of the unfolded protein response (UPR). Maladaptive UPR may explain the cerebellar and skeletal muscle degeneration responsible for the ataxia and muscle weakness typical of MSS. However, the cause of other more variable, clinical manifestations, such as mild to severe mental retardation, hypogonadism, short stature, and skeletal deformities, is less clear. To gain insights into the pathogenic mechanisms and/or adaptive responses to SIL1 loss, we carried out cell biological and proteomic investigations in skin fibroblasts derived from a young patient carrying the SIL1 R111X mutation. Despite fibroblasts not being overtly affected in MSS, we found morphological and biochemical changes indicative of UPR activation and altered cell metabolism. All the cell machineries involved in RNA splicing and translation were strongly downregulated, while protein degradation via lysosome-based structures was boosted, consistent with an attempt of the cell to reduce the workload of the endoplasmic reticulum and dispose of misfolded proteins. Cell metabolism was extensively affected as we observed a reduction in lipid synthesis, an increase in beta oxidation, and an enhancement of the tricarboxylic acid cycle, with upregulation of eight of its enzymes. Finally, the catabolic pathways of various amino acids, including valine, leucine, isoleucine, tryptophan, lysine, aspartate, and phenylalanine, were enhanced, while the biosynthetic pathways of arginine, serine, glycine, and cysteine were reduced. These results indicate that, in addition to UPR activation and increased protein degradation, MSS fibroblasts have profound metabolic alterations, which may help them cope with the absence of SIL1.
Keywords: BiP; autophagosome; fibroblast; neurodegenerative disease; pathway analysis; protein folding; unfolded protein response.
Conflict of interest statement
The authors declare no conflict of interest. The funder had no role in study design, data collection and analysis, the decision to publish, or preparation of the manuscript.
Figures



References
-
- Anttonen A.K. Marinesco-Sjogren Syndrome. In: Adam M.P., Ardinger H.H., Pagon R.A., Wallace S.E., Bean L.J.H., Mirzaa G., Amemiya A., editors. GeneReviews®. University of Washington; Seattle, WA, USA: 1993. - PubMed
-
- Roos A., Buchkremer S., Kollipara L., Labisch T., Gatz C., Zitzelsberger M., Brauers E., Nolte K., Schroder J.M., Kirschner J., et al. Myopathy in Marinesco-Sjogren syndrome links endoplasmic reticulum chaperone dysfunction to nuclear envelope pathology. Acta Neuropathol. 2014;127:761–777. doi: 10.1007/s00401-013-1224-4. - DOI - PubMed
-
- Filezac de L’Etang A., Maharjan N., Cordeiro Brana M., Ruegsegger C., Rehmann R., Goswami A., Roos A., Troost D., Schneider B.L., Weis J., et al. Marinesco-Sjogren syndrome protein SIL1 regulates motor neuron subtype-selective ER stress in ALS. Nat. Neurosci. 2015;18:227–238. doi: 10.1038/nn.3903. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

