Osteopontin Deficiency Ameliorates Prostatic Fibrosis and Inflammation
- PMID: 34830342
- PMCID: PMC8617904
- DOI: 10.3390/ijms222212461
Osteopontin Deficiency Ameliorates Prostatic Fibrosis and Inflammation
Abstract
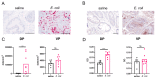
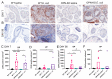
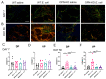
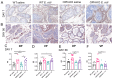
Fibrogenic and inflammatory processes in the prostate are linked to the development of lower urinary tract symptoms (LUTS) in men. Our previous studies identified that osteopontin (OPN), a pro-fibrotic cytokine, is abundant in the prostate of men with LUTS, and its secretion is stimulated by inflammatory cytokines potentially to drive fibrosis. This study investigates whether the lack of OPN ameliorates inflammation and fibrosis in the mouse prostate. We instilled uropathogenic E. coli (UTI89) or saline (control) transurethrally to C57BL/6J (WT) or Spp1tm1Blh/J (OPN-KO) mice and collected the prostates one or 8 weeks later. We found that OPN mRNA and protein expression were significantly induced by E. coli-instillation in the dorsal prostate (DP) after one week in WT mice. Deficiency in OPN expression led to decreased inflammation and fibrosis and the prevention of urinary dysfunction after 8 weeks. RNAseq analysis identified that E. coli-instilled WT mice expressed increased levels of inflammatory and fibrotic marker RNAs compared to OPN-KO mice including Col3a1, Dpt, Lum and Mmp3 which were confirmed by RNAscope. Our results indicate that OPN is induced by inflammation and prolongs the inflammatory state; genetic blockade of OPN accelerates recovery after inflammation, including a resolution of prostate fibrosis.
Keywords: benign prostatic hyperplasia; chronic inflammation; extracellular matrix; lower urinary tract symptoms; prostatic fibrosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


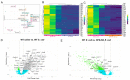
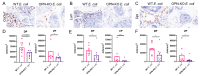
References
-
- Vuichoud C., Loughlin K.R. Benign prostatic hyperplasia: Epidemiology, economics and evaluation. Can. J. Urol. 2015;22:1–6. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

