The Role of Plant Hormones in the Interaction of Colletotrichum Species with Their Host Plants
- PMID: 34830343
- PMCID: PMC8620030
- DOI: 10.3390/ijms222212454
The Role of Plant Hormones in the Interaction of Colletotrichum Species with Their Host Plants
Abstract
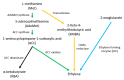
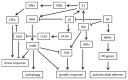
Colletotrichum is a plant pathogenic fungus which is able to infect virtually every economically important plant species. Up to now no common infection mechanism has been identified comparing different plant and Colletotrichum species. Plant hormones play a crucial role in plant-pathogen interactions regardless whether they are symbiotic or pathogenic. In this review we analyze the role of ethylene, abscisic acid, jasmonic acid, auxin and salicylic acid during Colletotrichum infections. Different Colletotrichum strains are capable of auxin production and this might contribute to virulence. In this review the role of different plant hormones in plant-Colletotrichum interactions will be discussed and thereby auxin biosynthetic pathways in Colletotrichum spp. will be proposed.
Keywords: Colletotrichum spp.; abscisic acid; auxin; ethylene; jasmonic acid; plant hormones; salicylic acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



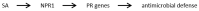

References
-
- Talhinhas P., Sreenivasaprasad S., Neves-Martins J., Oliveira H. Molecular and Phenotypic Analyses Reveal Association of Diverse Colletotrichum acutatum Groups and a Low Level of C. gloeosporioides with Olive Anthracnose. Appl. Environ. Microbiol. 2005;71:2987–2998. doi: 10.1128/AEM.71.6.2987-2998.2005. - DOI - PMC - PubMed
-
- Xavier K.V., Pfeiffer T., Parreira D.F., Chopra S., Vaillancourt L. Aggressiveness of Colletotrichum sublineola Strains from Sorghum bicolor and S. halepense to Sweet Sorghum Variety Sugar Drip, and Their Impact on Yield. Plant Dis. 2017;101:1578–1587. doi: 10.1094/PDIS-09-16-1238-RE. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

