Three-Dimensional Culture Systems for Dissecting Notch Signalling in Health and Disease
- PMID: 34830355
- PMCID: PMC8618738
- DOI: 10.3390/ijms222212473
Three-Dimensional Culture Systems for Dissecting Notch Signalling in Health and Disease
Abstract
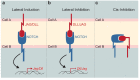
Three-dimensional (3D) culture systems opened up new horizons in studying the biology of tissues and organs, modelling various diseases, and screening drugs. Producing accurate in vitro models increases the possibilities for studying molecular control of cell-cell and cell-microenvironment interactions in detail. The Notch signalling is linked to cell fate determination, tissue definition, and maintenance in both physiological and pathological conditions. Hence, 3D cultures provide new accessible platforms for studying activation and modulation of the Notch pathway. In this review, we provide an overview of the recent advances in different 3D culture systems, including spheroids, organoids, and "organ-on-a-chip" models, and their use in analysing the crucial role of Notch signalling in the maintenance of tissue homeostasis, pathology, and regeneration.
Keywords: 3D culture systems; Notch signalling; cancer; drug screening; microfluidics; organ-on-a-chip; organoids; regeneration; spheroids; therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

