Isolation, Culture and Comprehensive Characterization of Biological Properties of Human Urine-Derived Stem Cells
- PMID: 34830384
- PMCID: PMC8624597
- DOI: 10.3390/ijms222212503
Isolation, Culture and Comprehensive Characterization of Biological Properties of Human Urine-Derived Stem Cells
Abstract
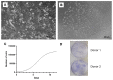
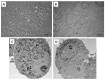
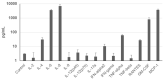
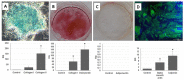
Mesenchymal stem cells (MSCs) represent an attractive source within the field of tissue engineering. However, their harvesting often requires invasive medical procedures. Urine-derived stem cells (UDSCs) display similar properties to MSCs, and their obtention and further processing is non-invasive for the donors as well as low cost. Here, we offer a comprehensive analysis of their biological properties. The goal of this study was to analyze their morphology, stemness, differentiation potential and cytokine profile. We have successfully isolated UDSCs from 25 urine samples. First colonies emerged up to 9 days after the initial seeding. Cell doubling time was 45 ± 0.24 SD, and when seeded at the density of 100 cells/cm2, they formed 42 ± 6.5 SD colonies within 10 days. Morphological analyzes revealed that two different types of the cell populations have been present. The first type had a rice-grain shape and the second one was characterized by a polyhedral shape. In several cell cultures, dome-shaped cells were observed as well. All examined UDSCs expressed typical MSC-like surface markers, CD73, CD90 and CD105. Moreover, conditioned media from UDSCs were harvested, and cytokine profile has been evaluated showing a significantly higher secretory rate of IL-8, IL-6 and chemokines MCP-1 and GM-CSF. We have also successfully induced human UDSCs into chondrogenic, osteogenic and myogenic cell lineages. Our findings indicate that UDSCs might have immense potential in the regeneration of the damaged tissues.
Keywords: biological characteristics; cytokine profile; differentiation capacity; morphology; stemness; urine-derived stem cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The human arthritic hip joint is a source of mesenchymal stromal cells (MSCs) with extensive multipotent differentiation potential.BMC Musculoskelet Disord. 2020 May 13;21(1):297. doi: 10.1186/s12891-020-03340-z. BMC Musculoskelet Disord. 2020. PMID: 32404085 Free PMC article.
-
Isolation and multilineage differentiation of bone marrow mesenchymal stem cells from abattoir-derived bovine fetuses.BMC Vet Res. 2013 Jul 5;9:133. doi: 10.1186/1746-6148-9-133. BMC Vet Res. 2013. PMID: 23826829 Free PMC article.
-
Effects of DLX3 on the osteogenic differentiation of induced pluripotent stem cell‑derived mesenchymal stem cells.Mol Med Rep. 2021 Apr;23(4):232. doi: 10.3892/mmr.2021.11871. Epub 2021 Jan 26. Mol Med Rep. 2021. PMID: 33655330 Free PMC article.
-
Alternative splicing in mesenchymal stem cell differentiation.Stem Cells. 2020 Oct 1;38(10):1229-1240. doi: 10.1002/stem.3248. Epub 2020 Jul 15. Stem Cells. 2020. PMID: 32627865 Free PMC article. Review.
-
Unravelling the role of interleukin-6 in regulating dental stem cell behaviour: a scoping review.BMC Oral Health. 2025 May 15;25(1):732. doi: 10.1186/s12903-025-06097-w. BMC Oral Health. 2025. PMID: 40375224 Free PMC article.
Cited by
-
Application prospects of urine-derived stem cells in neurological and musculoskeletal diseases.World J Orthop. 2024 Oct 18;15(10):918-931. doi: 10.5312/wjo.v15.i10.918. eCollection 2024 Oct 18. World J Orthop. 2024. PMID: 39473520 Free PMC article. Review.
-
Body fluid-derived stem cells - an untapped stem cell source in genitourinary regeneration.Nat Rev Urol. 2023 Dec;20(12):739-761. doi: 10.1038/s41585-023-00787-2. Epub 2023 Jul 6. Nat Rev Urol. 2023. PMID: 37414959 Free PMC article. Review.
-
Urine-derived stem cells serve as a robust platform for generating native or engineered extracellular vesicles.Stem Cell Res Ther. 2024 Sep 11;15(1):288. doi: 10.1186/s13287-024-03903-0. Stem Cell Res Ther. 2024. PMID: 39256816 Free PMC article.
-
Body Fluid-Derived Stem Cells: Powering Innovative, Less-Invasive Cell Therapies.Int J Mol Sci. 2025 May 5;26(9):4382. doi: 10.3390/ijms26094382. Int J Mol Sci. 2025. PMID: 40362618 Free PMC article. Review.
-
The Role of Extracellular Matrix and Hydrogels in Mesenchymal Stem Cell Chondrogenesis and Cartilage Regeneration.Life (Basel). 2022 Dec 9;12(12):2066. doi: 10.3390/life12122066. Life (Basel). 2022. PMID: 36556431 Free PMC article. Review.
References
-
- Chapple C. Overview on the Lower Urinary Tract. In: Andersson K.-E., Michel M.C., editors. Urinary Tract. Springer; Berlin, Germany: 2011. pp. 1–14.
-
- Corder C.J., Rathi B.M., Sharif S., Leslie S.W. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2021. 24-Hour Urine Collection. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

