Pro-Inflammatory Serum Amyloid a Stimulates Renal Dysfunction and Enhances Atherosclerosis in Apo E-Deficient Mice
- PMID: 34830462
- PMCID: PMC8623330
- DOI: 10.3390/ijms222212582
Pro-Inflammatory Serum Amyloid a Stimulates Renal Dysfunction and Enhances Atherosclerosis in Apo E-Deficient Mice
Abstract
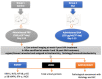
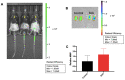
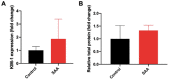
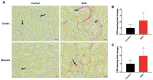
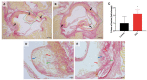
Acute serum amyloid A (SAA) is an apolipoprotein that mediates pro-inflammatory and pro-atherogenic pathways. SAA-mediated signalling is diverse and includes canonical and acute immunoregulatory pathways in a range of cell types and organs. This study aimed to further elucidate the roles for SAA in the pathogenesis of vascular and renal dysfunction. Two groups of male ApoE-deficient mice were administered SAA (100 µL, 120 µg/mL) or vehicle control (100 µL PBS) and monitored for 4 or 16 weeks after SAA treatment; tissue was harvested for biochemical and histological analyses at each time point. Under these conditions, SAA administration induced crosstalk between NF-κB and Nrf2 transcriptional factors, leading to downstream induction of pro-inflammatory mediators and antioxidant response elements 4 weeks after SAA administration, respectively. SAA treatment stimulated an upregulation of renal IFN-γ with a concomitant increase in renal levels of p38 MAPK and matrix metalloproteinase (MMP) activities, which is linked to tissue fibrosis. In the kidney of SAA-treated mice, the immunolocalisation of inducible nitric oxide synthase (iNOS) was markedly increased, and this was localised to the parietal epithelial cells lining Bowman's space within glomeruli, which led to progressive renal fibrosis. Assessment of aortic root lesion at the study endpoint revealed accelerated atherosclerosis formation; animals treated with SAA also showed evidence of a thinned fibrous cap as judged by diffuse collagen staining. Together, this suggests that SAA elicits early renal dysfunction through promoting the IFN-γ-iNOS-p38 MAPK axis that manifests as the fibrosis of renal tissue and enhanced cardiovascular disease.
Keywords: atherosclerosis; dysfunction; pro-inflammatory; renal; serum amyloid A.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Hashimoto K., Kataoka N., Nakamura E., Tsujioka K., Kajiya F. Oxidized LDL specifically promotes the initiation of monocyte invasion during transendothelial migration with upregulated PECAM-1 and downregulated VE-cadherin on endothelial junctions. Atherosclerosis. 2007;194:e9–e17. doi: 10.1016/j.atherosclerosis.2006.11.029. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

