Viral Myocarditis-From Pathophysiology to Treatment
- PMID: 34830522
- PMCID: PMC8623269
- DOI: 10.3390/jcm10225240
Viral Myocarditis-From Pathophysiology to Treatment
Abstract
The diagnosis of acute and chronic myocarditis remains a challenge for clinicians. Characterization of this disease has been hampered by its diverse etiologies and heterogeneous clinical presentations. Most cases of myocarditis are caused by infectious agents. Despite successful research in the last few years, the pathophysiology of viral myocarditis and its sequelae leading to severe heart failure with a poor prognosis is not fully understood and represents a significant public health issue globally. Most likely, at a certain point, besides viral persistence, several etiological types merge into a common pathogenic autoimmune process leading to chronic inflammation and tissue remodeling, ultimately resulting in the clinical phenotype of dilated cardiomyopathy. Understanding the underlying molecular mechanisms is necessary to assess the prognosis of patients and is fundamental to appropriate specific and personalized therapeutic strategies. To reach this clinical prerequisite, there is the need for advanced diagnostic tools, including an endomyocardial biopsy and guidelines to optimize the management of this disease. The severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) has currently led to the worst pandemic in a century and has awakened a special sensitivity throughout the world to viral infections. This work aims to summarize the pathophysiology of viral myocarditis, advanced diagnostic methods and the current state of treatment options.
Keywords: autoimmunity; dilated cardiomyopathy; endomyocardial biopsy; pathophysiology; therapeutic approach; viral myocarditis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



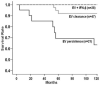
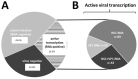


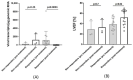

References
-
- Caforio A.L.P., Pankuweit S., Arbustini E., Basso C., Blanes J.G., Felix S.B., Fu M., Heliö T., Heymans S., Jahns R., et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013;34:2636–2648. doi: 10.1093/eurheartj/eht210. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

