Oncogenic KRAS: Signaling and Drug Resistance
- PMID: 34830757
- PMCID: PMC8616169
- DOI: 10.3390/cancers13225599
Oncogenic KRAS: Signaling and Drug Resistance
Abstract
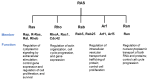
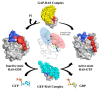
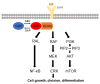
RAS proteins play a role in many physiological signals transduction processes, including cell growth, division, and survival. The Ras protein has amino acids 188-189 and functions as GTPase. These proteins are switch molecules that cycle between inactive GDP-bound and active GTP-bound by guanine nucleotide exchange factors (GEFs). KRAS is one of the Ras superfamily isoforms (N-RAS, H-RAS, and K-RAS) that frequently mutate in cancer. The mutation of KRAS is essentially performing the transformation in humans. Since most RAS proteins belong to GTPase, mutated and GTP-bound active RAS is found in many cancers. Despite KRAS being an important molecule in mostly human cancer, including pancreatic and breast, numerous efforts in years past have persisted in cancer therapy targeting KRAS mutant. This review summarizes the biological characteristics of these proteins and the recent progress in the exploration of KRAS-targeted anticancer, leading to new insight.
Keywords: GTPase; KRAS; drug resistance; inhibitor; mutant; signaling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Blockade of mutant RAS oncogenic signaling with a special emphasis on KRAS.Pharmacol Res. 2021 Oct;172:105806. doi: 10.1016/j.phrs.2021.105806. Epub 2021 Aug 24. Pharmacol Res. 2021. PMID: 34450320 Review.
-
KRAS: From undruggable to a druggable Cancer Target.Cancer Treat Rev. 2020 Sep;89:102070. doi: 10.1016/j.ctrv.2020.102070. Epub 2020 Jul 15. Cancer Treat Rev. 2020. PMID: 32711246 Review.
-
NMR in integrated biophysical drug discovery for RAS: past, present, and future.J Biomol NMR. 2020 Nov;74(10-11):531-554. doi: 10.1007/s10858-020-00338-6. Epub 2020 Aug 17. J Biomol NMR. 2020. PMID: 32804298 Review.
-
A KRAS GTPase K104Q Mutant Retains Downstream Signaling by Offsetting Defects in Regulation.J Biol Chem. 2017 Mar 17;292(11):4446-4456. doi: 10.1074/jbc.M116.762435. Epub 2017 Jan 30. J Biol Chem. 2017. PMID: 28154176 Free PMC article.
-
Mutant-Specific Targeting of Ras G12C Activity by Covalently Reacting Small Molecules.Cell Chem Biol. 2019 Oct 17;26(10):1338-1348. doi: 10.1016/j.chembiol.2019.07.005. Epub 2019 Aug 1. Cell Chem Biol. 2019. PMID: 31378709 Review.
Cited by
-
Polarization of Cancer-Associated Macrophages Maneuver Neoplastic Attributes of Pancreatic Ductal Adenocarcinoma.Cancers (Basel). 2023 Jul 5;15(13):3507. doi: 10.3390/cancers15133507. Cancers (Basel). 2023. PMID: 37444617 Free PMC article. Review.
-
Daily Practice Assessment of KRAS Status in NSCLC Patients: A New Challenge for the Thoracic Pathologist Is Right around the Corner.Cancers (Basel). 2022 Mar 23;14(7):1628. doi: 10.3390/cancers14071628. Cancers (Basel). 2022. PMID: 35406400 Free PMC article. Review.
-
Regulation of nucleotide excision repair by wild-type HRAS signaling in head and neck cancer.Cancer Gene Ther. 2025 Jun;32(6):662-677. doi: 10.1038/s41417-025-00902-y. Epub 2025 Apr 12. Cancer Gene Ther. 2025. PMID: 40221503 Free PMC article.
-
Identification of Synergistic Drug Combinations to Target KRAS-Driven Chemoradioresistant Cancers Utilizing Tumoroid Models of Colorectal Adenocarcinoma and Recurrent Glioblastoma.Front Oncol. 2022 May 18;12:840241. doi: 10.3389/fonc.2022.840241. eCollection 2022. Front Oncol. 2022. PMID: 35664781 Free PMC article.
-
Clinical and genetic drivers of oligo-metastatic disease in colon cancer.Neoplasia. 2025 Feb;60:101111. doi: 10.1016/j.neo.2024.101111. Epub 2024 Dec 21. Neoplasia. 2025. PMID: 39709701 Free PMC article.
References
-
- Merrick B.A., Phadke D.P., Bostrom M.A., Shah R.R., Wright G.M., Wang X., Gordon O., Pelch K.E., Auerbach S.S., Paules R.S. Arsenite malignantly transforms human prostate epithelial cells in vitro by gene amplification of mutated KRAS. PLoS ONE. 2019;14:e0215504. doi: 10.1371/journal.pone.0215504. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

