Head and Neck Cancers Are Not Alike When Tarred with the Same Brush: An Epigenetic Perspective from the Cancerization Field to Prognosis
- PMID: 34830785
- PMCID: PMC8616074
- DOI: 10.3390/cancers13225630
Head and Neck Cancers Are Not Alike When Tarred with the Same Brush: An Epigenetic Perspective from the Cancerization Field to Prognosis
Abstract
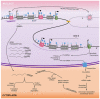
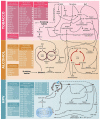
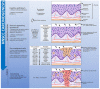
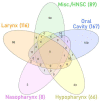
Head and neck squamous cell carcinomas (HNSCC) are among the ten most frequent types of cancer worldwide and, despite all efforts, are still diagnosed at late stages and show poor overall survival. Furthermore, HNSCC patients often experience relapses and the development of second primary tumors, as a consequence of the field cancerization process. Therefore, a better comprehension of the molecular mechanisms involved in HNSCC development and progression may enable diagnosis anticipation and provide valuable tools for prediction of prognosis and response to therapy. However, the different biological behavior of these tumors depending on the affected anatomical site and risk factor exposure, as well as the high genetic heterogeneity observed in HNSCC are major obstacles in this pursue. In this context, epigenetic alterations have been shown to be common in HNSCC, to discriminate the tumor anatomical subsites, to be responsive to risk factor exposure, and show promising results in biomarker development. Based on this, this review brings together the current knowledge on alterations of DNA methylation and microRNA expression in HNSCC natural history, focusing on how they contribute to each step of the process and on their applicability as biomarkers of exposure, HNSCC development, progression, and response to therapy.
Keywords: DNA methylation; HPV; alcohol; biomarker; field cancerization; head and neck cancer; microRNA; tobacco.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
Epigenetic Modifications and Head and Neck Cancer: Implications for Tumor Progression and Resistance to Therapy.Int J Mol Sci. 2017 Jul 12;18(7):1506. doi: 10.3390/ijms18071506. Int J Mol Sci. 2017. PMID: 28704968 Free PMC article. Review.
-
Human papillomavirus infection on initiating synchronous esophageal neoplasia in patients with head and neck cancer.Laryngoscope. 2016 May;126(5):1097-102. doi: 10.1002/lary.25728. Epub 2015 Nov 24. Laryngoscope. 2016. PMID: 27107411
-
Epigenetic Regulations of Perineural Invasion in Head and Neck Squamous Cell Carcinoma.Front Genet. 2022 Apr 27;13:848557. doi: 10.3389/fgene.2022.848557. eCollection 2022. Front Genet. 2022. PMID: 35571032 Free PMC article. Review.
-
Epigenetic Modifications in Head and Neck Cancer.Biochem Genet. 2020 Apr;58(2):213-244. doi: 10.1007/s10528-019-09941-1. Epub 2019 Nov 11. Biochem Genet. 2020. PMID: 31712935 Free PMC article. Review.
-
Telomeres and telomerase in head and neck squamous cell carcinoma: from pathogenesis to clinical implications.Cancer Metastasis Rev. 2016 Sep;35(3):457-74. doi: 10.1007/s10555-016-9633-1. Cancer Metastasis Rev. 2016. PMID: 27501725 Free PMC article. Review.
Cited by
-
The Next Chapter in Cancer Diagnostics: Advances in HPV-Positive Head and Neck Cancer.Biomolecules. 2024 Jul 30;14(8):925. doi: 10.3390/biom14080925. Biomolecules. 2024. PMID: 39199313 Free PMC article. Review.
-
It Takes Two to Tango: A Review of Oncogenic Virus and Host Microbiome Associated Inflammation in Head and Neck Cancer.Cancers (Basel). 2022 Jun 25;14(13):3120. doi: 10.3390/cancers14133120. Cancers (Basel). 2022. PMID: 35804891 Free PMC article. Review.
-
Oral Papillomatosis: Its Relation with Human Papilloma Virus Infection and Local Immunity-An Update.Medicina (Kaunas). 2022 Aug 15;58(8):1103. doi: 10.3390/medicina58081103. Medicina (Kaunas). 2022. PMID: 36013570 Free PMC article. Review.
-
Association between Periodontal Disease and Oral Benign, Potentially Malignant, Malignant, and Chronic Immune-Mediated Disorders: A Clinical Study.Healthcare (Basel). 2024 Oct 7;12(19):1999. doi: 10.3390/healthcare12191999. Healthcare (Basel). 2024. PMID: 39408179 Free PMC article.
-
Current and Emerging Diagnostic, Prognostic, and Predictive Biomarkers in Head and Neck Cancer.Biomedicines. 2024 Feb 10;12(2):415. doi: 10.3390/biomedicines12020415. Biomedicines. 2024. PMID: 38398017 Free PMC article. Review.
References
-
- Brierley J.D., Gospodarowicz M.K., Wittekind C. TNM Classification of Malignant Tumours. John Wiley & Sons; Hoboken, NJ, USA: 2017.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

