Microtubule Targeting Agents in Disease: Classic Drugs, Novel Roles
- PMID: 34830812
- PMCID: PMC8616087
- DOI: 10.3390/cancers13225650
Microtubule Targeting Agents in Disease: Classic Drugs, Novel Roles
Abstract
Microtubule-targeting agents (MTAs) represent one of the most successful first-line therapies prescribed for cancer treatment. They interfere with microtubule (MT) dynamics by either stabilizing or destabilizing MTs, and in culture, they are believed to kill cells via apoptosis after eliciting mitotic arrest, among other mechanisms. This classical view of MTA therapies persisted for many years. However, the limited success of drugs specifically targeting mitotic proteins, and the slow growing rate of most human tumors forces a reevaluation of the mechanism of action of MTAs. Studies from the last decade suggest that the killing efficiency of MTAs arises from a combination of interphase and mitotic effects. Moreover, MTs have also been implicated in other therapeutically relevant activities, such as decreasing angiogenesis, blocking cell migration, reducing metastasis, and activating innate immunity to promote proinflammatory responses. Two key problems associated with MTA therapy are acquired drug resistance and systemic toxicity. Accordingly, novel and effective MTAs are being designed with an eye toward reducing toxicity without compromising efficacy or promoting resistance. Here, we will review the mechanism of action of MTAs, the signaling pathways they affect, their impact on cancer and other illnesses, and the promising new therapeutic applications of these classic drugs.
Keywords: cancer; microtubule-targeting agent (MTA); microtubules (MTs); migration; pathogen; tauopathies; vascular formation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
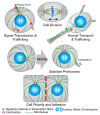
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

