Pimavanserin: A Novel Autophagy Modulator for Pancreatic Cancer Treatment
- PMID: 34830816
- PMCID: PMC8616166
- DOI: 10.3390/cancers13225661
Pimavanserin: A Novel Autophagy Modulator for Pancreatic Cancer Treatment
Abstract
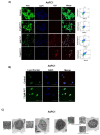
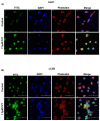
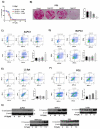
Pancreatic tumors exhibit high basal autophagy compared to that of other cancers. Several studies including those from our laboratory reported that enhanced autophagy leads to apoptosis in cancer cells. In this study, we evaluated the autophagy and apoptosis inducing effects of Pimavanserin tartrate (PVT). Autophagic effects of PVT were determined by Acridine Orange assay and Transmission Electron Microscopy analysis. Clinical significance of ULK1 in normal and pancreatic cancer patients was evaluated by R2 and GEPIA cancer genomic databases. Modulation of proteins in autophagy signaling was assessed by Western blotting and Immunofluorescence. Apoptotic effects of PVT was evaluated by Annexin-V/APC assay. Subcutaneous xenograft pancreatic tumor model was used to evaluate the autophagy-mediated apoptotic effects of PVT in vivo. Autophagy was induced upon PVT treatment in pancreatic ducal adenocarcinoma (PDAC) cells. Pancreatic cancer patients exhibit reduced levels of autophagy initiator gene, ULK1, which correlated with reduced patient survival. Interestingly, PVT induced the expression of autophagy markers ULK1, FIP200, Atg101, Beclin-1, Atg5, LC3A/B, and cleavage of caspase-3, an indicator of apoptosis in several PDAC cells. ULK1 agonist LYN-1604 enhanced the autophagic and apoptotic effects of PVT. On the other hand, autophagy inhibitors chloroquine and bafilomycin blocked the autophagic and apoptotic effects of PVT in PDAC cells. Notably, chloroquine abrogated the growth suppressive effects of PVT by 25% in BxPC3 tumor xenografts in nude mice. Collectively, our results indicate that PVT mediated pancreatic tumor growth suppression was associated with induction of autophagy mediated apoptosis.
Keywords: apoptosis; autophagy; cancer; cell death; drug repurposing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Repurposing Pimavanserin, an Anti-Parkinson Drug for Pancreatic Cancer Therapy.Mol Ther Oncolytics. 2020 Sep 2;19:19-32. doi: 10.1016/j.omto.2020.08.019. eCollection 2020 Dec 16. Mol Ther Oncolytics. 2020. PMID: 33024816 Free PMC article.
-
Pimavanserin tartrate induces apoptosis and cytoprotective autophagy and synergizes with chemotherapy on triple negative breast cancer.Biomed Pharmacother. 2023 Dec;168:115665. doi: 10.1016/j.biopha.2023.115665. Epub 2023 Oct 11. Biomed Pharmacother. 2023. PMID: 37832400
-
Endoplasmic reticulum stress sensitizes human esophageal cancer cell to radiation.World J Gastroenterol. 2013 Mar 21;19(11):1736-48. doi: 10.3748/wjg.v19.i11.1736. World J Gastroenterol. 2013. PMID: 23555162 Free PMC article.
-
Genistein potentiates the antitumor effect of 5-Fluorouracil by inducing apoptosis and autophagy in human pancreatic cancer cells.Anticancer Res. 2014 Sep;34(9):4685-92. Anticancer Res. 2014. PMID: 25202045 Free PMC article.
-
Therapeutic Targeting of Autophagy in Pancreatic Ductal Adenocarcinoma.Front Pharmacol. 2021 Nov 30;12:751568. doi: 10.3389/fphar.2021.751568. eCollection 2021. Front Pharmacol. 2021. PMID: 34916936 Free PMC article. Review.
Cited by
-
Serotonin signalling in cancer: Emerging mechanisms and therapeutic opportunities.Clin Transl Med. 2024 Jul;14(7):e1750. doi: 10.1002/ctm2.1750. Clin Transl Med. 2024. PMID: 38943041 Free PMC article. Review.
-
Does an apple a day keep away diseases? Evidence and mechanism of action.Food Sci Nutr. 2023 Jun 20;11(9):4926-4947. doi: 10.1002/fsn3.3487. eCollection 2023 Sep. Food Sci Nutr. 2023. PMID: 37701204 Free PMC article. Review.
-
Reprogramming tumor immune microenvironment by milbemycin oxime results in pancreatic tumor growth suppression and enhanced anti-PD-1 efficacy.Mol Ther. 2024 Sep 4;32(9):3145-3162. doi: 10.1016/j.ymthe.2024.07.029. Epub 2024 Aug 3. Mol Ther. 2024. PMID: 39097773 Free PMC article.
-
5-Hydroxytryptamine G-Protein-Coupled Receptor Family Genes: Key Players in Cancer Prognosis, Immune Regulation, and Therapeutic Response.Genes (Basel). 2024 Nov 28;15(12):1541. doi: 10.3390/genes15121541. Genes (Basel). 2024. PMID: 39766808 Free PMC article.
-
Autophagy and the pancreas: Healthy and disease states.Front Cell Dev Biol. 2024 Sep 24;12:1460616. doi: 10.3389/fcell.2024.1460616. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39381372 Free PMC article. Review.
References
-
- Khandia R., Dadar M., Munjal A., Dhama K., Karthik K., Tiwari R., Yatoo M.I., Iqbal H.M., Singh K.P., Joshi S.K., et al. A Comprehensive Review of Autophagy and Its Various Roles in Infectious, Non-Infectious, and Lifestyle Diseases: Current Knowledge and Prospects for Disease Prevention, Novel Drug Design, and Therapy. Cells. 2019;8:674. doi: 10.3390/cells8070674. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

