The Classification of Myeloproliferative Neoplasms: Rationale, Historical Background and Future Perspectives with Focus on Unclassifiable Cases
- PMID: 34830822
- PMCID: PMC8616346
- DOI: 10.3390/cancers13225666
The Classification of Myeloproliferative Neoplasms: Rationale, Historical Background and Future Perspectives with Focus on Unclassifiable Cases
Abstract
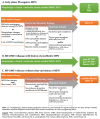
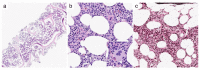
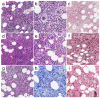
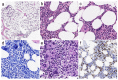
Myeloproliferative neoplasms (MPNs) are a heterogeneous group of clonal hematopoietic stem cell disorders, characterized by increased proliferation of one or more myeloid lineages in the bone marrow. The classification and diagnostic criteria of MPNs have undergone relevant changes over the years, reflecting the increased awareness on these conditions and a better understanding of their biological and clinical-pathological features. The current World Health Organization (WHO) Classification acknowledges four main sub-groups of MPNs: (i) Chronic Myeloid Leukemia; (ii) classical Philadelphia-negative MPNs (Polycythemia Vera; Essential Thrombocythemia; Primary Myelofibrosis); (iii) non-classical Philadelphia-negative MPNs (Chronic Neutrophilic Leukemia; Chronic Eosinophilic Leukemia); and (iv) MPNs, unclassifiable (MPN-U). The latter are currently defined as MPNs with clinical-pathological findings not fulfilling the diagnostic criteria for any other entity. The MPN-U spectrum traditionally encompasses early phase MPNs, terminal (i.e., advanced fibrotic) MPNs, and cases associated with inflammatory or neoplastic disorders that obscure the clinical-histological picture. Several lines of evidence and clinical practice suggest the existence of additional myeloid neoplasms that may expand the spectrum of MPN-U. To gain insight into such disorders, this review addresses the history of MPN classification, the evolution of their diagnostic criteria and the complex clinical-pathological and biological features of MPN-U.
Keywords: MPN-U; Myeloproliferative neoplasms; WHO Classification; myeloid disorders.
Conflict of interest statement
The Authors declare no conflict of interest.
Figures

References
-
- Swerdlow S.H., Campo E., Harris N.L., Jaffe E.S., Pileri S.A., Stein H., Thiele J. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press; Lyon, France: 2017.
-
- Szuber N., Mudireddy M., Nicolosi M., Penna D., Vallapureddy R.R., Lasho T.L., Finke C., Begna K.H., Elliott M.A., Hook C.C., et al. 3023 Mayo Clinic Patients with Myeloproliferative Neoplasms: Risk-Stratified Comparison of Survival and Outcomes Data Among Disease Subgroups. Mayo Clin. Proc. 2019;94:599–610. doi: 10.1016/j.mayocp.2018.08.022. - DOI - PubMed

