Blood Cytokine Analysis Suggests That SARS-CoV-2 Infection Results in a Sustained Tumour Promoting Environment in Cancer Patients
- PMID: 34830872
- PMCID: PMC8616215
- DOI: 10.3390/cancers13225718
Blood Cytokine Analysis Suggests That SARS-CoV-2 Infection Results in a Sustained Tumour Promoting Environment in Cancer Patients
Abstract
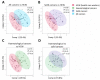
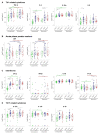
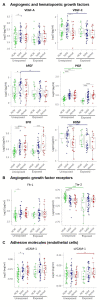
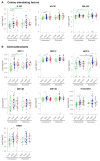
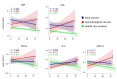
Cytokines, chemokines, and (angiogenic) growth factors (CCGs) have been shown to play an intricate role in the progression of both solid and haematological malignancies. Recent studies have shown that SARS-CoV-2 infection leads to a worse outcome in cancer patients, especially in haematological malignancy patients. Here, we investigated how SARS-CoV-2 infection impacts the already altered CCG levels in solid or haematological malignancies, specifically, whether there is a protective effect or rather a potentially higher risk for major COVID-19 complications in cancer patients due to elevated CCGs linked to cancer progression. Serially analysing immune responses with 55 CCGs in cancer patients under active treatment with or without SARS-CoV-2 infection, we first showed that cancer patients without SARS-CoV-2 infection (n = 54) demonstrate elevated levels of 35 CCGs compared to the non-cancer, non-infected control group of health care workers (n = 42). Of the 35 CCGs, 19 were common to both the solid and haematological malignancy groups and comprised previously described cytokines such as IL-6, TNF-α, IL-1Ra, IL-17A, and VEGF, but also several less well described cytokines/chemokines such as Fractalkine, Tie-2, and T cell chemokine CTACK. Importantly, we show here that 7 CCGs are significantly altered in SARS-CoV-2 exposed cancer patients (n = 52). Of these, TNF-α, IFN-β, TSLP, and sVCAM-1, identified to be elevated in haematological cancers, are also known tumour-promoting factors. Longitudinal analysis conducted over 3 months showed persistence of several tumour-promoting CCGs in SARS-CoV-2 exposed cancer patients. These data demonstrate a need for increased vigilance for haematological malignancy patients as a part of long COVID follow-up.
Keywords: COVID-19; Th1; Th17; Th2; anti-inflammatory; haematological cancers; immune response; pro-inflammatory; solid cancers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

