FTIR Spectroscopic Imaging Supports Urine Cytology for Classification of Low- and High-Grade Bladder Carcinoma
- PMID: 34830887
- PMCID: PMC8616357
- DOI: 10.3390/cancers13225734
FTIR Spectroscopic Imaging Supports Urine Cytology for Classification of Low- and High-Grade Bladder Carcinoma
Abstract
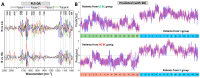
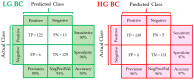
Bladder urothelial carcinoma (BC) is a common, recurrent, life-threatening, and unpredictable disease which is difficult to diagnose. These features make it one of the costliest malignancies. Although many possible diagnostic methods are available, molecular heterogeneity and difficulties in cytological or histological examination induce an urgent need to improve diagnostic techniques. Herein, we applied Fourier transform infrared spectroscopy in imaging mode (FTIR) to investigate patients' cytology samples assigned to normal (N), low-grade (LG) and high-grade (HG) BC. With unsupervised hierarchical cluster analysis (UHCA) and hematoxylin-eosin (HE) staining, we observed a correlation between N cell types and morphology. High-glycogen superficial (umbrella) and low-glycogen piriform urothelial cells, both with normal morphology, were observed. Based on the spectra derived from UHCA, principal component analysis (PCA) and partial least squares discriminant analysis (PLS-DA) were performed, indicating a variation of protein content between the patient groups. Moreover, BC spectral cytology identified a low number of high-glycogen cells for which a shift of the carbohydrate/phosphate bands was also observed. Despite high cellular heterogeneity, PLS-DA was able to classify the spectra obtained. The voided urine FTIR cytology is one of the options that might be helpful in BC diagnosis, as high sensitivity and specificity up to 97% were determined.
Keywords: bladder carcinoma; cytology; diagnostics; infrared spectroscopic imaging.
Conflict of interest statement
The authors declare no competing interest.
Figures



Similar articles
-
Cytokeratin 20 immunocytochemistry on urine sediments: A potential low-cost adjunct to cytology in the diagnosis of low-grade urothelial carcinoma.Cytopathology. 2017 Dec;28(6):531-535. doi: 10.1111/cyt.12463. Epub 2017 Sep 22. Cytopathology. 2017. PMID: 28940433
-
Spectrochemical differentiation of meningioma tumours based on attenuated total reflection Fourier-transform infrared (ATR-FTIR) spectroscopy.Anal Bioanal Chem. 2020 Feb;412(5):1077-1086. doi: 10.1007/s00216-019-02332-w. Epub 2019 Dec 21. Anal Bioanal Chem. 2020. PMID: 31865413 Free PMC article.
-
Is urinary tract cytology still useful for diagnosis of bladder carcinomas? A large series of 592 bladder washings using a five-category classification of different cytological diagnoses.Cytopathology. 2007 Apr;18(2):79-83. doi: 10.1111/j.1365-2303.2007.00426.x. Cytopathology. 2007. PMID: 17397491
-
Current status of urinary cytology in the evaluation of bladder neoplasms.Hum Pathol. 1990 Sep;21(9):886-96. doi: 10.1016/0046-8177(90)90171-z. Hum Pathol. 1990. PMID: 2203672 Review.
-
Genitourinary cytopathology (kidney and urinary tract).Cancer Treat Res. 2014;160:149-83. doi: 10.1007/978-3-642-38850-7_7. Cancer Treat Res. 2014. PMID: 24092370 Review.
Cited by
-
Detection of Sialic Acid to Differentiate Cervical Cancer Cell Lines Using a Sambucus nigra Lectin Biosensor.Biosensors (Basel). 2024 Jan 10;14(1):34. doi: 10.3390/bios14010034. Biosensors (Basel). 2024. PMID: 38248411 Free PMC article.
-
Raman Spectroscopy as a Potential Adjunct of Thyroid Nodule Evaluation: A Systematic Review.Int J Mol Sci. 2023 Oct 13;24(20):15131. doi: 10.3390/ijms242015131. Int J Mol Sci. 2023. PMID: 37894812 Free PMC article.
-
Translating Biospectroscopy Techniques to Clinical Settings: A New Paradigm in Point-of-Care Screening and/or Diagnostics.J Pers Med. 2023 Oct 19;13(10):1511. doi: 10.3390/jpm13101511. J Pers Med. 2023. PMID: 37888122 Free PMC article.
References
-
- Koss L.G., Hoda R.S. Koss’s Cytology of the Urinary Tract with Histopathologic Correlations. Springer; Berlin/Heidelberg, Germany: 2012.
Grants and funding
LinkOut - more resources
Full Text Sources

