Radiolabeled Bombesin Analogs
- PMID: 34830920
- PMCID: PMC8616220
- DOI: 10.3390/cancers13225766
Radiolabeled Bombesin Analogs
Abstract
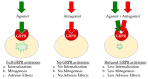
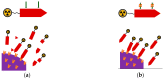
The gastrin-releasing peptide receptor (GRPR) is expressed in high numbers in a variety of human tumors, including the frequently occurring prostate and breast cancers, and therefore provides the rationale for directing diagnostic or therapeutic radionuclides on cancer lesions after administration of anti-GRPR peptide analogs. This concept has been initially explored with analogs of the frog 14-peptide bombesin, suitably modified at the N-terminus with a number of radiometal chelates. Radiotracers that were selected for clinical testing revealed inherent problems associated with these GRPR agonists, related to low metabolic stability, unfavorable abdominal accumulation, and adverse effects. A shift toward GRPR antagonists soon followed, with safer analogs becoming available, whereby, metabolic stability and background clearance issues were gradually improved. Clinical testing of three main major antagonist types led to promising outcomes, but at the same time brought to light several limitations of this concept, partly related to the variation of GRPR expression levels across cancer types, stages, previous treatments, and other factors. Currently, these parameters are being rigorously addressed by cell biologists, chemists, nuclear medicine physicians, and other discipline practitioners in a common effort to make available more effective and safe state-of-the-art molecular tools to combat GRPR-positive tumors. In the present review, we present the background, current status, and future perspectives of this endeavor.
Keywords: cancer theranostics; gastrin-releasing peptide receptor; radiolabeled bombesin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




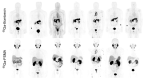
References
-
- Jensen R.T., Battey J.F., Spindel E.R., Benya R.V. International union of pharmacology. LXVIII. Mammalian bombesin receptors: Nomenclature, distribution, pharmacology, signaling, and functions in normal and disease states. Pharmacol. Rev. 2008;60:1–42. doi: 10.1124/pr.107.07108. - DOI - PMC - PubMed
-
- Markwalder R., Reubi J.C. Gastrin-releasing peptide receptors in the human prostate: Relation to neoplastic transformation. Cancer Res. 1999;59:1152–1159. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

