Dual-Wavelength Fluorescence Monitoring of Photodynamic Therapy: From Analytical Models to Clinical Studies
- PMID: 34830963
- PMCID: PMC8616416
- DOI: 10.3390/cancers13225807
Dual-Wavelength Fluorescence Monitoring of Photodynamic Therapy: From Analytical Models to Clinical Studies
Abstract
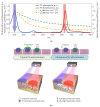
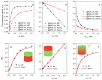
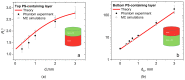
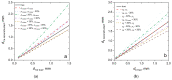
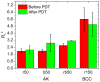
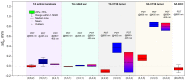
Fluorescence imaging modalities are currently a routine tool for the assessment of marker distribution within biological tissues, including monitoring of fluorescent photosensitizers (PSs) in photodynamic therapy (PDT). Conventional fluorescence imaging techniques provide en-face two-dimensional images, while depth-resolved techniques require complicated tomographic modalities. In this paper, we report on a cost-effective approach for the estimation of fluorophore localization depth based on dual-wavelength probing. Owing to significant difference in optical properties of superficial biotissues for red and blue ranges of optical spectra, simultaneous detection of fluorescence excited at different wavelengths provides complementary information from different measurement volumes. Here, we report analytical and numerical models of the dual-wavelength fluorescence imaging of PS-containing biotissues considering topical and intravenous PS administration, and demonstrate the feasibility of this approach for evaluation of the PS localization depth based on the fluorescence signal ratio. The results of analytical and numerical simulations, as well as phantom experiments, were translated to the in vivo imaging to interpret experimental observations in animal experiments, human volunteers, and clinical studies. The proposed approach allowed us to estimate typical accumulation depths of PS localization which are consistent with the morphologically expected values for both topical PS administration and intravenous injection.
Keywords: Monte Carlo simulations; animal studies; chlorin-based photosensitizers; clinical studies; dual-wavelength fluorescence imaging; light transport; optical phantoms; photodynamic therapy.
Conflict of interest statement
The authors declare no competing interests.
Figures
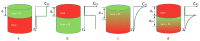





References
-
- Bao X., Yuan Y., Chen J., Zhang B., Li D., Zhou D., Jing P., Xu G., Wang Y., Holá K. In vivo theranostics with near-infrared-emitting carbon dots—highly efficient photothermal therapy based on passive targeting after intravenous administration. Light Sci. Appl. 2018;7:1–11. doi: 10.1038/s41377-018-0090-1. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

