Gfi1 Loss Protects against Two Models of Induced Diabetes
- PMID: 34831029
- PMCID: PMC8616283
- DOI: 10.3390/cells10112805
Gfi1 Loss Protects against Two Models of Induced Diabetes
Abstract
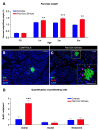
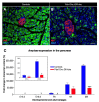
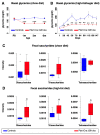
Background: Although several approaches have revealed much about individual factors that regulate pancreatic development, we have yet to fully understand their complicated interplay during pancreas morphogenesis. Gfi1 is transcription factor specifically expressed in pancreatic acinar cells, whose role in pancreas cells fate identity and specification is still elusive. Methods: In order to gain further insight into the function of this factor in the pancreas, we generated animals deficient for Gfi1 specifically in the pancreas. Gfi1 conditional knockout animals were phenotypically characterized by immunohistochemistry, RT-qPCR, and RNA scope. To assess the role of Gfi1 in the pathogenesis of diabetes, we challenged Gfi1-deficient mice with two models of induced hyperglycemia: long-term high-fat/high-sugar feeding and streptozotocin injections. Results: Interestingly, mutant mice did not show any obvious deleterious phenotype. However, in depth analyses demonstrated a significant decrease in pancreatic amylase expression, leading to a diminution in intestinal carbohydrates processing and thus glucose absorption. In fact, Gfi1-deficient mice were found resistant to diet-induced hyperglycemia, appearing normoglycemic even after long-term high-fat/high-sugar diet. Another feature observed in mutant acinar cells was the misexpression of ghrelin, a hormone previously suggested to exhibit anti-apoptotic effects on β-cells in vitro. Impressively, Gfi1 mutant mice were found to be resistant to the cytotoxic and diabetogenic effects of high-dose streptozotocin administrations, displaying a negligible loss of β-cells and an imperturbable normoglycemia. Conclusions: Together, these results demonstrate that Gfi1 could turn to be extremely valuable for the development of new therapies and could thus open new research avenues in the context of diabetes research.
Keywords: amylase; ghrelin; high-fat diet; mouse model; pancreas development.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- ElSayed S.A., Mukherjee S. Physiology, Pancreas. StatPearls Publishing; Treasure Island, FL, USA: 2018.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

